Techno-Economic Evaluation of Novel SARS-CoV-2 Vaccine Manufacturing in the Insect Cell Baculovirus Platform
Corresponding email: kanya.alifia@gmail.com
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6139
Alifia, K.C.H., Kontoravdi, C.Kis, Z., Ismail, D., 2022. Techno-Economic Evaluation of Novel SARS-CoV-2 Vaccine Manufacturing in the Insect Cell Baculovirus Platform. International Journal of Technology. Volume 13(8), pp. 1630-1639
| Kanya Citta Hani Alifia | 1. Department of Chemical Engineering, ACE Extension, Faculty of Engineering, Imperial College London, South Kensington Campus, London SW7 2AZ, United Kingdom, 2. Department of Chemical Engineering, |
| Cleo Kontoravdi | Department of Chemical Engineering, ACE Extension, Faculty of Engineering, Imperial College London, South Kensington Campus, London SW7 2AZ, United Kingdom |
| Zoltán Kis | Department of Chemical and Biological Engineering, Sir Robert Hadfield Building, The University of Sheffield, Mappin Street, Sheffield, S1 3JD, United Kingdom |
| Dianursanti Ismail | Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424 Indonesia |
The need to increase the COVID-19 vaccine
manufacturing capacity at low to middle-income countries (LMIC) led to a
growing focus on Novavax (NVX-CoV2373), a thermostable protein subunit vaccine
manufactured using a baculovirus and insect cell system (BICS) platform. This
study aimed to conduct a techno-economic analysis to assess the BICS platform
of vaccine manufacturing and compare it to the mRNA and the saRNA platform. The
data from the Novavax patent for the COVID-19 vaccine formulation and the
manufacturing steps were used to simulate the BICS vaccine production in
SuperPro Designer. From the techno-economic analysis, the productivity of all
platforms was compared in terms of doses/day per L production scale. The saRNA
platform’s productivity is about 1,000-fold of the BICS platform and 20-fold of
the mRNA platform. BICS is a feasible option for LMIC to produce vaccines
because the cost per dose is like the saRNA platform, while the mRNA platform’s
cost per dose is 7 times higher than the BICS and saRNA platforms. However,
further optimization is necessary to improve the productivity of the BICS
platform to match saRNA’s platform.
Baculovirus; COVID-19; Insect cell; Techno-economic analysis; Vaccine
The
advancement of modern
technology enabled researchers to predict the properties of the COVID-19 virus
and apply this knowledge to rapidly develop vaccines that successfully lessened the impact of the COVID-19
pandemic globally as posited by Berawi et al.
(2020a, 2020b). As of 2nd August 2022, 5.3 billion people had
received at least one dose of vaccine, which accounts for 67% of the world
population. In total, 12.36 billion vaccine doses have been administered
worldwide (Ritchie et al., 2021). However,
there is a clear gap in vaccination rates among countries. High-income
countries can administer 100 doses per 100 people on average whereas low-income
countries had not even reached a 20% vaccination rate by August 2022 (Ritchie et al., 2021; Irwin, 2021). The
manufacturing capacity of COVID-19 vaccines in 2021 was around 8 billion doses
a year; a combined capacity of AstraZeneca, Pfizer, Sinovac, Sinopharm, and
Moderna (AstraZeneca, 2021; Pfizer, 2021; Shumei, 2021; Steenhuysen &
O'Donnell, 2021). Although global herd immunity was achieved due to
vaccinations, some low-income countries failed to achieve this (Ritchie et al., 2021). As COVID-19 virus rapidly
mutated into different variants throughout the years, this highlights the
importance of annual booster shots in the future, which adds to the vaccine
demands (O'Neill, 2021). Thus, it is
necessary to increase the vaccine manufacturing capacity mainly aimed for low
to middle-income countries (LMIC).
RNA vaccines such as Pfizer
and Moderna require ultra-cold storage at -70°C and -20°C, respectively (Gerhardt et al., 2021). The distribution of these
vaccines is challenging in warmer areas where access to an ultra-cold supply
chain is insufficient. Other vaccine types such as inactivated whole virus,
viral vector, and protein subunit only require 2-8°C temperature for storage in
a refrigerator, with Novavax (2021a, 2021b) as
the leading example. Novavax (NVX-CoV2373) is a protein subunit vaccine with
89.3% efficacy against multiple variants in its phase 3 trial conducted in the
UK (Novavax, 2021a). It is manufactured
using a baculovirus and insect cell system (BICS) platform. A platform
technology can be used to manufacture various vaccines simply by modifying the
genetic sequence of the cloned baculovirus. Adopting a platform technology will
improve the resilience capacity of biopharmaceutical industries to be prepared
against future pandemics. Vaccine production using platform technology have
more robust and rapid productivity. Moreover, a platform technology is
adjustable to produce different vaccines (Sofyan et al., 2021).
BICS is a well-established
platform for vaccine manufacturing using recombinant DNA technology, it can
produce three different vaccine types: recombinant proteins as subunit
vaccines, virus-like particles (VLPs) as subunit vaccines, and recombinant
baculovirus as vaccine vectors. The vaccine development process starts with
modifying the recombinant baculovirus to contain the gene of interest from the
native virus. This gene can either encode the formation of protein subunits,
the construction of VLPs, or produce antigens to be carried by the baculovirus
vectors (Mena & Kamen, 2011). The insect
cells act as the host, which contains the necessary organelles for heterologous
protein production and can rapidly construct the desired component (Sari et al., 2016). Insect cells have a higher
reproduction rate than mammalian cells and contain a protein folding mechanism
that bacteria lack, highlighting the advantage of the BICS platform (Mena & Kamen, 2011).
To make Novavax vaccines,
the genetic sequence of SARS-CoV-2 spike protein is cloned into baculovirus
culture to infect Sf9 insect cells for the protein folding process. The
expressed antigen protein is then purified as multimeric nanoparticles and
configured with saponin-based Matrix-M™ adjuvant to enhance neutralizing
antibodies and increase long-lasting B-cell and T-cell immunity (Novavax, 2021b). This vaccine is thermostable,
adaptable to new COVID-19 variants, feasible for rapid large-scale production,
and can be produced with standard equipment (Novavax,
2016 & 2021c). Novavax released its patent for SARS-CoV-2 vaccine
formulation in March 2021, showing the manufacturing steps and the trials that
were taken to determine the optimum formulation of antigen substance and
adjuvant (Novavax, 2021c). This patent was
used to build the vaccine production flowsheet with SuperPro Designer software;
a process
simulator that facilitates the modelling, evaluation, and optimization of
integrated biological and chemical processes. Meanwhile the mass
balances in the bioreactors are calculated by accounting for the insect cells
metabolic fluxes to estimate the stoichiometric reaction equation (Carinhas et al., 2011; Gioria et al., 2006).
It is beneficial to compare
Novavax’s insect cell vaccine with the mRNA (messenger RNA) vaccine and novel
saRNA (self-amplifying RNA) vaccine, to find out which platform can achieve the
target vaccine cost per dose of 1 USD (Kis et al.,
2020a). A comparative study of
various vaccine platforms had been commenced with the indicators such as
technology readiness, complexity, ease of scale-up, flexibility, vaccine
thermostability, and speed of response. These indicators show that RNA and BICS
platforms are nearly up to par, but a more detailed feasibility study must be
done with techno-economic analysis (Kis et al.,
2019). Moreover, Kis et
al. also conducted a techno-economic simulation of the mRNA and saRNA vaccine
platform, which will be the benchmark for BICS platform performance (Kis et al., 2020b). This study aimed to
conduct a techno-economic analysis to assess the BICS platform for COVID-19
vaccine manufacturing using SuperPro Designer software and compare it with
previous findings.
A literature review was conducted to
gather information regarding COVID-19 vaccine production processes in the BICS
platform. The step-by-step production process and the costs are taken from Novavax patents (2016, 2021c), scientific
literature (Kis et al., 2020a; Kis et al., 2019; Sari et al., 2016; Mena & Kamen,
2011), and trusted suppliers such as ThermoFisher, Sigma Aldrich, Cytiva
Life Sciences, and GE Life Sciences. The production flowsheet was designed
according to the block flow diagram of CoV-S protein vaccine production in BICS
from the Novavax patent, especially for the parameters of the bioreactor and
downstream processes (Novavax, 2021c; Kis et al.,
2019). Additional data was obtained from the SuperPro Designer
equipment, materials, utilities, and cost databases. The demand for BICS
vaccines was estimated at 3 billion doses, considering by 2021 that 8 out of 11
billion doses had been met by existing manufacturers.
2.1. Simulation of COVID-19 Vaccine Production
in BICS Platform
The vaccine production
process was modeled using SuperPro Designer version 12 from Intelligen, Inc
starting from the upstream, midstream, until downstream assuming fed-batch
operation mode. The formulation and the fill-to-finish line were not simulated
in SuperPro Designer since it is usually done in a separate facility. This
bioprocess simulation tool can calculate the material and energy balances,
equipment sizes, labor requirements, and optimal scheduling of operations and
procedures. SuperPro Designer version 12 can also procure an economic
evaluation using its built-in database, user-specified costs, and selling
prices (Canizales et al., 2020).
For the
upstream and midstream processes, the cultivation of Hi-5 insect cells is done
using a 5-500 L disposable bioreactor and then scaled up into a 2000 L seed
bioreactor, while the virus amplification is done in a separate line with Sf-9
insect cells in 5-500 L disposable bioreactors. In the next step, the
baculovirus transfects the insect cells in a 2500 L bioreactor to instruct the
cells to express the spike protein antigen of the SARS-CoV-2 virus (Novavax, 2021c; Kis et al., 2019). The duration of cell culture in the main production bioreactor
lasts 48-96 hours (Novavax, 2021c). The
stoichiometric reaction equation for the Sf9 insect cell growth phase, the Hi5
insect cell growth phase, and the baculovirus infection phase are modelled
according to the metabolic fluxes of the cells (Carinhas
et al., 2011; Gioria et al., 2006). This equation is necessary to model
the mass balances inside the bioreactors.
The downstream
separation step starts with centrifugation to separate the cells from the
liquid medium, then mixed with Triton X-100 for cell lysis. Then the mixture is
passed through the microfiltration step to separate the antigen polypeptides
from cell debris. Polypeptide nanoparticles are formed using a detergent
exchange method in a sequence of affinity chromatography, where the first
column uses NP9 detergent, and the second column uses PS80 detergent. The
result will be trimers of polypeptides or glycoproteins attached to a detergent
core. For the downstream purification, the mixture undergoes dialysis of CoV-S
polypeptide in a solution of sodium phosphate, NaCl, and PS80, as well as
ultrafiltration. The mixture is frozen until it is ready for the formulation
step in another facility, to be mixed with excipients and Matrix-M™ adjuvant.
The CoV-S polypeptide drug substance per vaccine ranges between 5-45 µg/dose
based on the clinical trial (Novavax, 2021c).
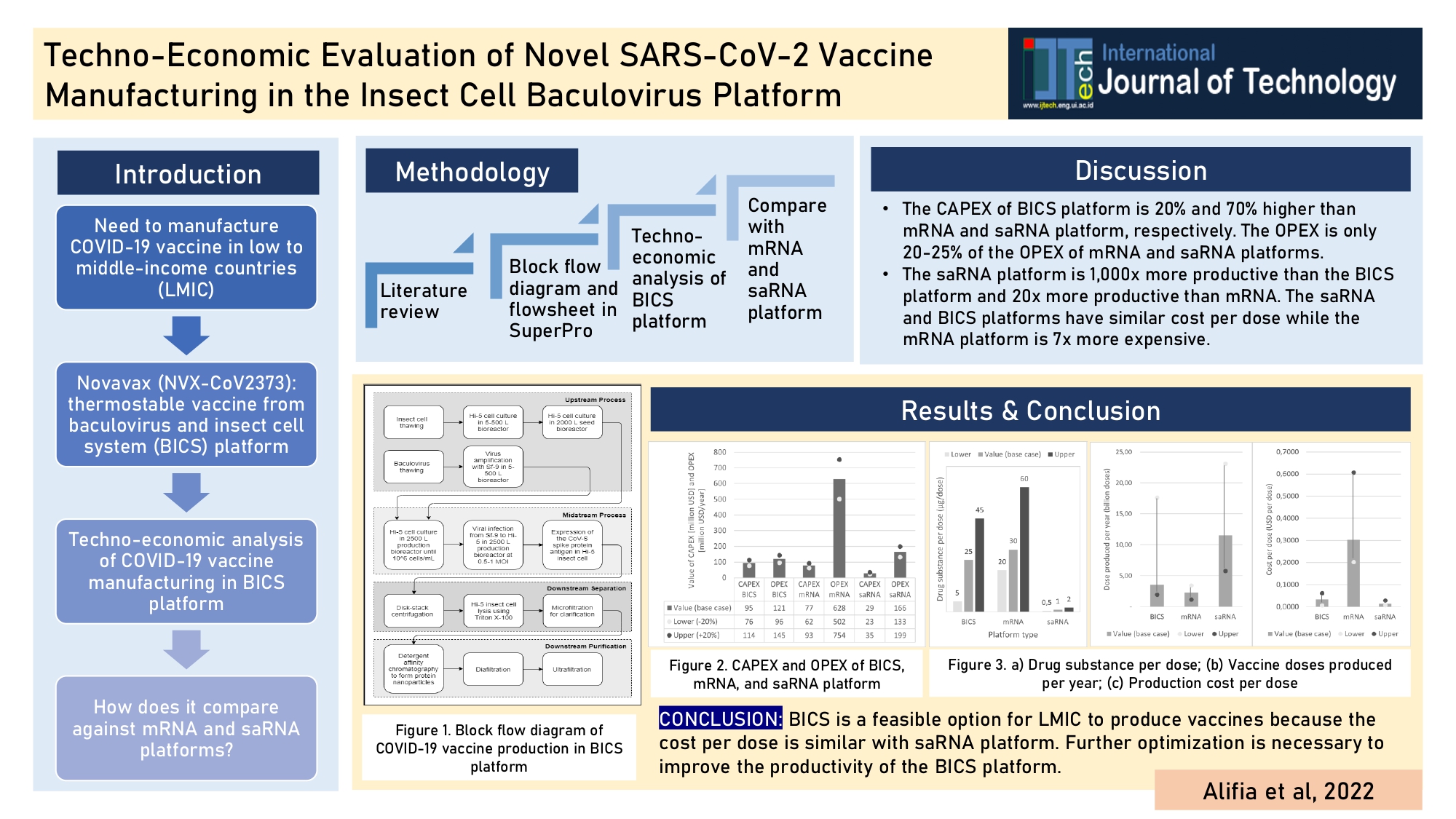
The block flow diagram of COVID-19 vaccine production in the BICS platform is
shown below (Figure 1). This diagram only shows the production of the CoV-S
spike protein antigen, which is the active ingredient in COVID-19 vaccines.
Further processing, such as formulation and packaging, are typically conducted
in a different plant, which are not accounted for in this simulation flowsheet.
Figure 1 Block flow diagram of
COVID-19 vaccine production in baculovirus-incest cell system (BICS)
2.2. Simulation of
COVID-19 Vaccine Production in mRNA and saRNA Platform
The
simulation for both platforms was done in a previous study by Kis et al. (2020b). In the upstream process, the
DNA template is generated, amplified in E. coli culture, purified, and
linearized. In the midstream process, the RNA is synthesized with in vitro
transcription reaction and 5’ cap analogs are used for the 5’ capping of the
RNA to ensure antigen expression. It is then purified and formulated in lipid
nanoparticles or polycationic formulations to maintain its stability. The
operation runs as a batch process that lasts 11 hours from start to finish. For
self-amplifying RNA (saRNA) vaccines, each dose contains 0.1-10 µg drug
substance while for mRNA vaccines each dose contains 25-250 µg drug substance (Kis et
al., 2019). The result from this study was compared to the techno-economic
analysis results of the BICS platform.
3.1. Techno-Economic Analysis
of BICS, mRNA, and saRNA Platform
A comparison of
techno-economic analysis between BICS and mRNA platforms is conducted to assess
which platform can fulfill global vaccine demands at the lowest cost possible.
The novel saRNA platform is also compared against these two platforms due to its
rapid production rate with less than 10 L reactor size (Kis
et al., 2019), showing potential for high-level productivity. The Phase
I clinical trial showed 87% effectiveness in 192 people aged 18-45, thus more
studies are needed to assess the safety and immunogenicity of saRNA vaccine in
other age groups and with a higher number of test subjects (Pollock et al., 2022).
The base case was calculated
at the median value of all process input parameters (process scale, process
failure rate, production titer, basic labor rate, CoV-S protein amount per
dose, and cost of lab/QC/QA) which then resulted in a particular production titer,
while the lower and upper case were calculated using ± 20% margin of the
production titer differences from the 10.5 g/L base case. The upper-case
scenario is when the process produces +20% production titer or 12.6 g/L, while
the lower-case scenario is when the process makes -20% production titer or 8.4
g/L (Kis et al., 2020b). The
summary of process input parameter values for the BICS simulation is shown in
Table 1.
Table 1 Input parameters and their respective ranges,
central values, and distribution
|
Parameter name and unit |
Value of input parameter |
Reference |
|
Process scale [L] |
2,500 |
(Novavax, 2021c) |
|
Process failure rate [%] |
2,500 |
(Novavax, 2021c) |
|
Production titre [g L-1] |
10.5 |
(Novavax, 2021c) |
|
Basic labor rate [USD hour 1] |
20 |
(Petrides, 2021) |
|
CoV-S protein amount per dose [µg dose-1] |
25 |
(Novavax, 2021c) |
|
Cost of Lab/QC/QA [% of total labor costs] |
40 |
(Petrides, 2021) |
3.1.1 Comparison
of CAPEX and OPEX
The capital expenditure
(CAPEX) and annual operating expenditure (OPEX) were calculated by SuperPro
Designer and then compared between BICS, mRNA, and saRNA platforms (Figure 2).
Figure 2 CAPEX and OPEX of vaccine production in BICS, mRNA,
and saRNA platform
The BICS platform has the
highest value of capital expnditure (CAPEX) at 76-114 million USD, followed by
the mRNA platform at 62-93 million USD, then the saRNA platform at 23-35 million
USD. The high CAPEX in the BICS platform is due to the significantly higher
production scale. The process also involves an upstream process of the Hi-5
insect cell culture line and virus amplification line in the Sf-9 insect cell,
which does not exist in RNA-based vaccine production. The main contributors to
the CAPEX in the BICS platform are equipment purchase, installation,
engineering, and construction fees.
The CAPEX of the mRNA
platform is 20% lower than the CAPEX of the BICS platform, while the CAPEX of
the saRNA platform is 70% lower than the CAPEX of the BICS platform. The
smaller production scale of RNA platforms reduced the cost of equipment
purchase and installation costs, engineering fees, and construction fees. The
CAPEX of the mRNA platform mainly consists of the buildings and construction costs
because the downstream process for mRNA vaccine drug substance requires
multiple steps such as tangential flow filtration (TFF), chromatography,
microfiltration, and dialysis, thus still needing enough space in the plant
layout (Petrides, 2021). The equipment cost
and installation cost are still lower because of the reduced scale compared to
the BICS platform. The main contributor of CAPEX in the saRNA platform is the
same as the mRNA platform due to the high similarity of the production process
in both platforms.
The annual operational
expenditure (OPEX) of mRNA platform is at 502-754 million USD. That is about 4
times larger than the OPEX of the saRNA platform at 133-199 million USD and
even 5 times larger than the OPEX of the BICS platform at 96-145 million USD.
The main reason the OPEX of the mRNA platform is very high is the raw material
cost, mainly the CleanCapAU priced at 340,000 USD per kg and the UTP priced at
230,000 USD per kg, which contribute to 35% and 24% of the total raw material
costs, respectively. With a price this high, it will be helpful to research any
substitute material or find ways to produce these at a lower cost. Using
single-use equipment for storage, mixing, and production of drug substances
also adds to the consumable costs. Overall, the raw materials and consumables
costs are 74% and 24% of the total OPEX, respectively.
3.1.2.
Comparison of cost per dose and productivity
The amount of drug substance
per dose varied based on the clinical trials of each vaccine type as shown in
Figure 3a (Novavax, 2021c; Kis et al., 2020b). The variations of scenarios would affect the
number of doses produced per year and correspond to the lower case, base case,
and upper case. The cost per dose is calculated by dividing the annual OPEX by
the annual doses produced. The yearly doses produced and the cost per dose are
presented in Figure 3b and Figure 3c. The production scale of BICS, mRNA, and
saRNA platforms are set at 2500 L, 30 L, and 7 L, respectively (Novavax, 2021c; Kis et al., 2020b). The prices for adjuvants (Matrix M and saponin) are considered
additional costs that increase the cost per dose (SigmaAldrich,
2021).
Figure 3 a) Drug substance per
dose; (b) Vaccine doses produced per year; (c) Production cost per dose
The productivity of each platform was obtained by dividing the doses
produced per year by the working days (assumed to be 330 days), then dividing
it again with the production scale volume. This calculation used the base
values from the range of inputs and the practical working days to obtain the
productivity value. The saRNA platform’s productivity is about 1,000-fold of
the BICS platform and 20-fold of the mRNA platform. The saRNA and BICS
platforms are economically up to par according to the cost per dose, while the
mRNA platform’s cost per dose is 7 times higher than the BICS and saRNA
platforms. These are shown in Table 2.
Table 2 Productivity of BICS, mRNA, and saRNA vaccine
production platform presented as doses per day per L bioreactor
|
Platform |
Doses/year |
Doses/day |
Production scale (L) |
Productivity (Doses/day per L) |
|
BICS |
±3,500,000,000 |
±11,000,000 |
2,500 |
±4,000 |
|
mRNA |
±2,300,000,000 |
±7,000,000 |
30 |
±200,000 |
|
saRNA |
±11,500,000,000 |
±35,000,000 |
7 |
±5,000,000 |
The smaller production scale of saRNA
compared to the mRNA platform significantly reduced the volume of CleanCapAU
needed thus reducing its OPEX. The mRNA platform has a higher throughput per
year at 69.04 kg of the drug substance while the saRNA platform only produced
11.55 kg of the drug substance. Comparing the production cost per kilogram
product shows the value for the saRNA platform at 14.4 million USD per kg
and the mRNA platform at 10.1 million
USD per kg. BICS platform remained the most economically feasible option at 1.3
million USD per kg product and 92.66 kg of the drug substance annual
throughput.
The OPEX of the BICS platform
is mainly dominated by the cost of baculovirus and insect cells (both Hi-5 and
Sf-9) as well as the consumables cost such as the disposable bioreactor and
Capto Lentil Lectin column (3400 GBP for 5 x 5 mL set) for the chromatography
process (SigmaAldrich, 2022; ThermoFisher, 2022; SigmaAldrich, 2021; Cytiva, 2020).
With the reproductive nature of insect cell culture, the cell line and scaling
up process do not require a lot of new cells and are only necessary to maintain
the insect cell culture in an optimum reactor condition and medium content. The
large production scale provides a higher annual throughput of 23 kg than the
mRNA platform. The base case for drug substance per dose for BICS and mRNA is
quite similar, thus their annual dose produced is only 1 billion doses apart.
With a significantly lower OPEX per kilogram drug substance than mRNA and
saRNA, the BICS platform can manufacture vaccines as rapidly as mRNA with an
almost 90% cheaper production cost per dose. Although saRNA has the highest
production cost per kilogram product at 14.4 million USD per kg, the ultra-low
dosage at 1 µg/dose enables the platform to produce 11.5 billion doses
annually. This drives the cost per dose even lower than the BICS platform.
The
smaller productivity (doses/day per L production scale) in the BICS platform is
due to the vast difference in production scale between the BICS platform and
both RNA-based platforms. The substantially lower amount of RNA drug substance
per dose for the saRNA vaccine also contributed to more rapid production of
vaccine doses compared to mRNA and BICS. When investing in a vaccine
manufacturing platform, there will be a trade-off to consider between platform
productivity and the cost per dose (Kis et al.,
2020b). When saRNA is ready for large-scale manufacturing after multiple
phases of clinical trials and assessment of current Good Manufacturing
Practices (cGMP), it will be fair to consider this platform for vaccine
production. Overall, the BICS platform shows a significant advantage over the
mRNA platform both technologically (annual production of vaccine doses) and
economically (cost per dose). The lower productivity will be a challenge for
further research on optimizing the production scale and productivity.
Baculovirus and insect cell system (BICS),
mRNA, and saRNA platforms were evaluated for their techno-economic feasibility
to manufacture the SARS-CoV-2 vaccine rapidly using SuperPro Designer. From the
techno-economic analysis, the saRNA
platform’s productivity is about 1,000-fold of the BICS platform and 20-fold of
the mRNA platform. The saRNA and BICS platforms are economically up to
par, as shown by their similar cost per dose, while the mRNA platform’s cost
per dose is 7 times higher than BICS and saRNA platforms. However, it is best
to focus on developing the BICS platform for SARS-CoV-2 vaccine manufacturing
in LMICs because it is more clinically developed than saRNA, which by 2021 had
not reached the clinical trials step while BICS had passed its third clinical
trial. Further research is needed to consider other costs in the
techno-economic analysis and optimization study of the BICS platform to improve
its productivity and lower its capital cost.
The
authors would like to acknowledge the Department of Chemical Engineering in
Imperial College London for facilitating this research in 2021. The authors
would like to thank the Research Center for Biomedical Engineering at the
University of Indonesia for conducting the joint conference of ACB-ISBE which
allowed the authors to present and publish this research. The authors are
thankful for the research Matching Fund scheme of “Hibah PUTI Q2” under the
Agreement Letter number: NKB-1470/UN2.RST/HKP.05.00/2022.
AstraZeneca,
2021. Pushing Boundaries to Deliver COVID-19 Vaccine Across
the Globe. Available Online at:
https://www.astrazeneca.com/what-science-can-do/topics/technologies/pushing-boundaries-to-deliver-covid-19-vaccine-accross-the-globe.html,
Accessed on December 20, 2021
Berawi, M.A.,
2020. Empowering Healthcare, Economic, and Social Resilience during Global
Pandemic Covid-19. International
Journal of Technology, Volume 11(3),
pp. 436–439
Berawi, M.A., Suwartha, N., Kusrini, E.,
Yuwono, A.H., Harwahyu, R., Setiawan, E.A., Yatmo, Y.A., Atmodiwirjo, P.,
Zagloel, Y.T., Suryanegara, M., Putra, N., Budiyanto, M.A., Whulanza, Y., 2020.
Tackling the COVID-19 Pandemic: Managing the Cause, Spread, and Impact. International
Journal of Technology, Volume 11(2), pp. 209–214
Canizales, L., Rojas, F., Pizarro, C.A., Caicedo-Ortega, N.H.,
Villegas-Torres, M.F., 2020. SuperPro Designer®, User-Oriented
Software Used for Analyzing the Techno-Economic Feasibility of Electrical
Energy Generation from Sugarcane Vinasse in Colombia. Processes,
Volume 8(9), p. 1180
Carinhas, N., Bernal, V., Teixeira,
A.P., Carrondo, M.J., Alves, P.M., Oliveira, R., 2011. Hybrid Metabolic Flux
Analysis: Combining Stoichiometric and Statistical Constraints to Model the
Formation of Complex Recombinant Products. In: BMC systems biology, Volume 5(1), pp.1–13
Cytiva, 2020.
Capto Lentil Lectin Affinity Chromatography Resin. Available Online at:
https://www.cytivalifesciences.com/en/us/shop/chromatography/resins/affinity-specific-groups/capto-lentil-lectin-affinity-chromatography-resin-p-05955,
Accessed on January 3, 2022
Gerhardt, A., Voigt, E., Archer, M.,
Reed, S., Larson, E., Van Hoeven, N., Kramer, R., Fox, C., Casper, C., 2021. A Thermostable,
Flexible RNA Vaccine Delivery Platform for Pandemic Response. In: bioRxiv.
DOI: https://doi.org/10.1101/2021.02.01.429283
Gioria, V.V., Jäger, V., Claus, J.D.,
2006. Growth, Metabolism and Baculovirus Production in Suspension Cultures of an
Anticarsia Gemmatalis Cell Line. In: Cytotechnology, Volume 52(2), pp.113–124
Irwin, A., 2021.
What It Will Take to Vaccinate The World Against COVID-19.
Available Online at: https://www.nature.com/articles/d41586-021-00727-3,
Accessed on December 20, 2021
Kis, Z.,
Kontoravdi, C., Dey, A., Shattock, R., Shah, N., 2020. Rapid Development and
Deployment of High?Volume Vaccines for Pandemic Response. Journal of Advanced Manufacturing and
Processing, Volume 2(3), p.e10060
Kis, Z., Kontoravdi, C., Shattock, R., Shah,
N., 2020. Resources, Production Scales and Time Required for Producing RNA Vaccines
for The Global Pandemic Demand. Vaccines, Volume 9(1), p. 3
Kis, Z., Shattock, R., Shah, N., Kontoravdi,
C., 2019. Emerging Technologies for Low?Cost, Rapid Vaccine Manufacture.
Biotechnology journal, Volume 14(1), p.1800376
Mena, J.A., Kamen,
A., 2011. Insect Cell Technology is a Versatile and Robust
Vaccine Manufacturing Platform. Expert Review of Vaccines, Volume 10(7), pp. 1063–1081
Novavax, 2016. Immunogenic
Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Compositions and
Methods. US Patent, No. 0206729 A1, July 21, 2016
Novavax, 2021a. Coronavirus
Vaccine Formulations. US Patent, No. 10953089 B1, March 23, 2021.
Novavax, 2021b. Novavax
COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial. Available
Online at: https://ir.novavax.com/2021-01-28-Novavax-COVID-19-Vaccine-Demonstrates-89-3-Efficacy-in-UK-Phase-3-Trial#:~:text=(Nasdaq%3A%20NVAX)%2C%20a,the%20United%20Kingdom%20(%20UK%20),
Accessed on December 24, 2021
Novavax, 2021c. Our Recombinant
Protein-Based Nanoparticle Vaccine Technology. Available Online at:
https://www.novavax.com/science-technology/recombinant-protein-based-nanoparticle-vaccine-technology#recombinant-%20nanoparticle-vaccine-technology,
Accessed on December 24, 2021
O'Neill, L.,
2021. Coronavirus: Will Immunity Rapidly Fade Or Last
A Lifetime?. Available Online at:
https://theconversation.com/coronavirus-will-immunity-rapidly-fade-or-last-a-lifetime-155905,
Accessed on January 12, 2022
Petrides, D.,
2021. SuperPro Designer User Guide - A Comprehensive
Simulation Tool for the Design, Retrofit & Evaluation of Specialty
Chemical, Biochemical, Pharmaceutical, Consumer Product, Food, Agricultural,
Mineral Processing, Packaging and Water Purification, Wastewater. Available
Online at:
http://www.intelligen.com/downloads/SuperPro_ManualForPrinting_v10.pdf, Accessed
on January 1, 2022
Pfizer, 2021. Manufacturing
and Distributing the COVID-19 Vaccine. Available Online at:
https://www.pfizer.com/science/coronavirus/vaccine/manufacturing-and-distribution,
Accessed on December 22,
2021
Pollock, K.M., Cheeseman, H.M., Szubert,
A.J., Libri, V., Boffito, M., Owen, D., Bern, H., O'Hara, J., McFarlane, L.R.,
Lemm, N.M., McKay, P.F., 2022. Safety and Immunogenicity of a Self-Amplifying RNA
Vaccine Against COVID-19: COVAC1, A Phase I, Dose-Ranging Trial. EClinicalMedicine, Volume 44, p.101262
Ritchie, H., Mathieu, E., Rodés-Guirao,
L., Appel, C., Giattino, C., Ortiz-Ospina, E., Hasell, J., Macdonald, B.,
Beltekian, D., Roser, M., 2021. A Global Database of COVID-19 Vaccinations. Nature
Human Behaviour, Volume 5, pp. 947–953
Sari, D., Gupta, K., Raj, D.B.T.G.,
Aubert, A., Drncová, P., Garzoni, F., Fitzgerald, D., Berger, I., 2016. The
MultiBac Baculovirus/Insect Cell Expression Vector System for Producing Complex
Protein Biologics. Advanced Technologies for Protein Complex
Production and Characterization, pp.
199–215
Shumei, L., 2021.
Sinovac to Lift Yearly Production Capacity to 2 Billion
Doses by June as China Expands Vaccination Program. Available Online at:
https://www.globaltimes.cn/page/202103/1217214.shtml, Accessed on January 5,
2022
SigmaAldrich,
2021. Matrix M and Saponin Adjuvant Price. Available Online at:
https://www.sigmaaldrich.com/GB/en/product/sigma/47036?gclid=Cj0KCQjwssyJBhDXARIsAK98ITRNziWVEv_51eil5Y9cwNQx6W8r8eCFdGww7Dq2uMmsvro7n7naXYaAryjEALw_wcB,
Accessed on January 10, 2022
SigmaAldrich,
2022. Sf9 Insect Cell Price. Available Online at:
https://www.sigmaaldrich.com/GB/en/product/mm/71104m?context=product, Accessed
on January 4, 2022
Sofyan, N.,
Yuwono, A.H., Harjanto, S., Budiyanto, M.A., Wulanza, Y., Putra, N.,
Kartohardjono, S., Kusrini, E., Berawi, M.A., Suwartha, N., Maknun, I.J.,
Yatmo, Y.A., Atmodiwirjo, P., Asvial, M., Harwahyu, R., Suryanegara, M.,
Setiawan, E.A., Zagloel, T.Y.M., Surjandari, I., 2021. Resilience and
Adaptability for a Post-Pandemic World: Exploring Technology to Enhance
Environmental Sustainability. International
Journal of Technology, Volume 12(6),
pp. 1091–1100
Steenhuysen, J.,
O'Donnell, C., 2021. Moderna Boosting COVID-19 Vaccine Capacity,
Targets Up to 3 Billion Shots in 2022.
Available Online at:
https://www.reuters.com/business/healthcare-pharmaceuticals/moderna-boosting-covid-19-vaccine-making-capacity-targets-up-3-billion-shots-2021-04-29/,
Accessed on January 9, 2022
ThermoFisher,
2022. Hi5 Insect Cell Price. Available Online at:
https://www.thermofisher.com/order/catalog/product/10486025#/10486025, Accessed
on January 4, 2022
