Methylenetetrahydrofolate Reductase (MTHFR) C677T and A1298C Gene Polymorphism as Risk Factors for Essential Hypertension
Corresponding email: asmarinah.si@gmail.com
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6138
Pratamawati, T.M., Alwi, I., Asmarinah, 2022. Methylenetetrahydrofolate Reductase (MTHFR) C677T and A1298C Gene Polymorphism as Risk Factors for Essential Hypertension. International Journal of Technology. Volume 13(8), pp. 1622-1629
| Tiar Masykuroh Pratamawati | 1. Doctoral Program in Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Kampus UI Salemba, 10430, Indonesia, 2. Department of Genetics, Faculty of Medicine Universitas Swadaya Gunung |
| Idrus Alwi | Division of Cardiology, Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia/Cipto Mangunkusumo National General Hospital, Kampus UI Salemba, 10430, Indonesia |
| Asmarinah Asmarinah | Department of Medical Biology, Faculty of Medicine, Universitas Indonesia, Kampus UI Salemba, 10430, Indonesia |
Hypertension has relatively large morbidity and mortality rates
throughout the world, including in Indonesia. The prevalence of hypertension
tends to be greater in patients with a family history of hypertension. This is
thought to be influenced by polymorphisms in the methylenetetrahydrofolate reductase
(MTHFR) gene. This study
aims to determine the relationship between the polymorphism of C677T and the A1298C MTHFR gene as a risk factor for essential hypertension. An
observational study with a case-control design was conducted involving 37 cases and 30 control people. Data obtained by PCR-RFLP. Data analysis was performed using chi-square and odds ratio calculations. The most common genotype for C677T
polymorphism is CC (94.6%) followed by CT and TT with 2.7% each (p = 0.001)
with OR of 0.099 (CI95% = 0.02-0.49). The most common genotype for the A1298C
polymorphism is AC (45.9%), followed by AA (35.1%) and CC (19%) (p = 0.001).
The C allele is present in 24 subjects in the case group (64.8%) and in 7
subjects in the control group (23.3%). The OR for the A1298C is 6.06 (CI 95% =
2.1-17.9). The C677T polymorphism showed statistical significance but did not
modify the risk factor of essential hypertension. Whereas the A1298C
polymorphism is statistically significant and has a 6-fold risk factor for
essential hypertension, polymorphism A1298C Methyltetrahydrofolate Reductase (MTHFR) gene is a risk factor of essential
hypertension.
A1298C; C677T; Essential hypertension; MTHFR gene; Polymorphism
Essential hypertension is a serious disease that is
spread worldwide and can be a severe complication such as stroke, heart attack,
retinopathy, and renal failure. Hypertension is a condition when a person's
systolic blood pressure (SBP) is ?140 mm Hg and diastolic blood pressure (DBP)
is ?90 mm Hg following repeated examination (Unger et al.,
2020). According to the Indonesian Ministry of Health,
hypertension can be classified into primary hypertension (90% of the case) and
secondary hypertension (10% of the case (P2PTM Kementerian Kesehatan, 2019). Hypertension
is the leading cause of cardiovascular diseases and the leading cause of death
from stroke and ischemic heart
Patients with essential
hypertension often present with a wide range of symptoms, including headache,
irregular heart rhythm, vision changes, chest pain, nausea, vomiting, and
convulsion. Currently, essential hypertension is diagnosed by measuring blood
pressure twice using a digital blood pressure monitor with an interval of 5
minutes. If the patient is confirmed for high blood pressure, a follow-up test includes electrocardiography and blood glucose
levels to rule out other cardiovascular diseases and diabetes as a cause.
Molecular testing for essential hypertension risk is not commonly done in
Indonesia, despite the added information it can provide for identifying risk
early. Therapy for essential hypertension includes diuretics,
angiotensin-converting enzyme inhibitors or angiotensin receptor blockers,
beta-blockers, and calcium channel blockers.
Hypertension risk factors that
cannot be modified include age, sex, and genetics, while modifiable risk
factors include smoking, low-fiber diet, dyslipidemia, salt over-consumption,
sedentary lifestyle, stress obesity, and alcohol consumption (Kemenkes RI, 2019).
Several genes have been
elucidated as a risk factor for hypertension, but genetic differences between
populations greatly affect the outcome (Huang et al., 2015). Genes studied as risk factors for hypertension
include the Angiotensin Converting Enzyme (ACE) gene, eNOS gene,
Renin-Angiotensin System gene, etc. (Shi et al.,
2021; Dhanachandra-Singh et al.,
2014; Choudhury et al., 2012). In addition, the MTHFR gene is
associated with cardiovascular disorders such as spina bifida, acute leukemia,
nutritional deficiency and down syndrome, and premature coronary artery
disease. , rheumatoid heart disease and hypertension ( Ward et al., 2020; Zaghloul et al., 2019; Kedar & Chandel,
2019; Carlus et al., 2016; Wilson et
al., 2013; Stover et al., 2015; Cortese & Motti, 2001; Wiemels et al., 2001)
Methylenetetrahydrofolate
reductase is an enzyme in the methyl cycle expressed by the MTHFR gene. This
gene is located in chromosome 1p36.3 at the base pair of 11.785.730-11.806.103.
Methylenetetrahydrofolate reductase catalyzes 5,10-methylenetetra hydrofolate to 5-methyltetrahydrofolate, a co-substrate for homocvsteine re-methvlation to methionine. Polymorphism in MTHFR is suspected to be one of the causes of elevated homocysteine, which can lead to increased blood pressure (Leclerc et al., 2013). Genetic research to see the genetic contribution to the phenotype of hypertension cases so that it can have implications for management (Padmanabhan et al., 2015). In addition, it is also known that internal factors such as genetics contribute to the occurrence of primary hypertension. Polymorphisms in hundreds of genes are associated with risk factors for hypertension (Evangelou et al., 2018). This research analyzes the possible involvement of MTHFR C677T and A1298C polymorphisms with essential hypertension. Both polymorphisms have been demonstrated to be involved in developing essential hypertension in various populations but are unknown in the Indonesian population. Genetic markers for essential hypertension can help first-line screening for risk factors in disease development.
2.1. Patient
Selection
An
analytical study with a case-control design was conducted in the medical
faculty of Swadaya Gunung Jati University, Indonesia, to assess the association
between Methyltetrahydrofolate Reductase (MTHFR) C677T And A1298C gene
polymorphism and essential hypertension. The target population was essential
hypertensive patients. We used the purposive sampling method.
The
inclusion criteria were all diagnosed with essential hypertensive and ECG
normal. Subjects with the following condition were excluded, i.e.: (1) patients
with any secondary cause of hypertension; (2) patients with abnormal ECG (4)
patients who disagreed with giving blood for the study. Control subjects with
the following condition, i.e.: (1) patient with normal blood pressure; (2)
patient with normal ECG. (Figure 1) This study was approved by the Ethical
Committee of the Faculty of Medicine, Universitas Swadaya Gunung Jati, Cirebon,
Indonesia. All patients had signed written informed consent prior to the study.
Written informed consent was
obtained from all participants prior to enrolment. A written consent was
obtained from their parents or guardians for underaged patients. The
Institutional Review Board of Faculty of Medicine Universitas Swadaya Gunung
Jati, Cirebon, Indonesia, approved the study protocol and followed the ethical
principles of the Declaration of Helsinki of 1975 and its revision.
2.2. Blood Collection & DNA Extraction
Blood
samples were collected from the peripheral vein. Then, they were put in
EDTA-coated tubes and kept cold. QIAamp kit (Qiagen, Tokyo, Japan) extracted
the DNA from leukocytes according to the standard protocol.
2.3. Genetic Analysis
PCR-RFLP for C677T with forward primers 5'TGAAGGAGAAGGTGTCTGCGGGA3' and reverse 5'AGGACGGTGCGGTGAG AGTG3', A1298C with forward primer 5'CAAGGAGGAGCTGCTGAAGA3' and reverse 5'CCACTCCAGCATCACTCACT3'. PCR was conducted using the following settings: C677T initial denaturation at 95 °C for 5 min. Thirty-five cycles of denaturation at 94°C for 30 sec; annealing at 60°C for 30 sec, extension at 2°C for 30 sec, and final extension at 2°C for 5 min. PCR settings for A1298C: initial denaturation at 94°C for 4 min, 30 cycles of denaturation at 94°C for 60 sec, annealing at 60°C for 60 sec, extension at 2°C for 60 sec, and final extension at 2°C for 10 min. The C677T PCR product was then digested using Hinfl and visualized with 1% agarose gel. The Al298C PCR product was subsequently digested using MboII and visualized with 1% agarose gel (Pratamawati et al., 2018).
Figure 1 Work-flow
schematics
This research recruited 34
male patients and 33 female patients, with an average age in the subject's age
of 40.30 years old and in the control group of 40.50 years (Table 1). MTHFR
C677T genotype distribution in the case group is CC (n=35, 94.6%), CT (n=1,
2.7%), and TT (n=1, 2.7%), and in the control group CC (n=19, 63.4%), CT (n=10,
33.3%), and TT (n=1, 2.7%). The distribution of the C allele is 71 (95.9%) in
the case group and 48 in the control group (80%), while the T allele
distribution is 3 (4.1%) in the case group and 12 (20%) in the control group
(Table 2).
MTHFR A1298C in the case group is AA
(n=13, 35.1%), AC (n=17, 45.9%), and AC (n=7, 19%), and in the control group AA
(n=23, 76.7%), AC (n=13.3%), and CC (n=3, 10%). The distribution of the A allele
is 43 (58.1%) in the case group and 50 in the control group (83.3%). The
distribution of the C allele in the case group is 43 (58.1%) and 50 in the
control group (83.3.%).
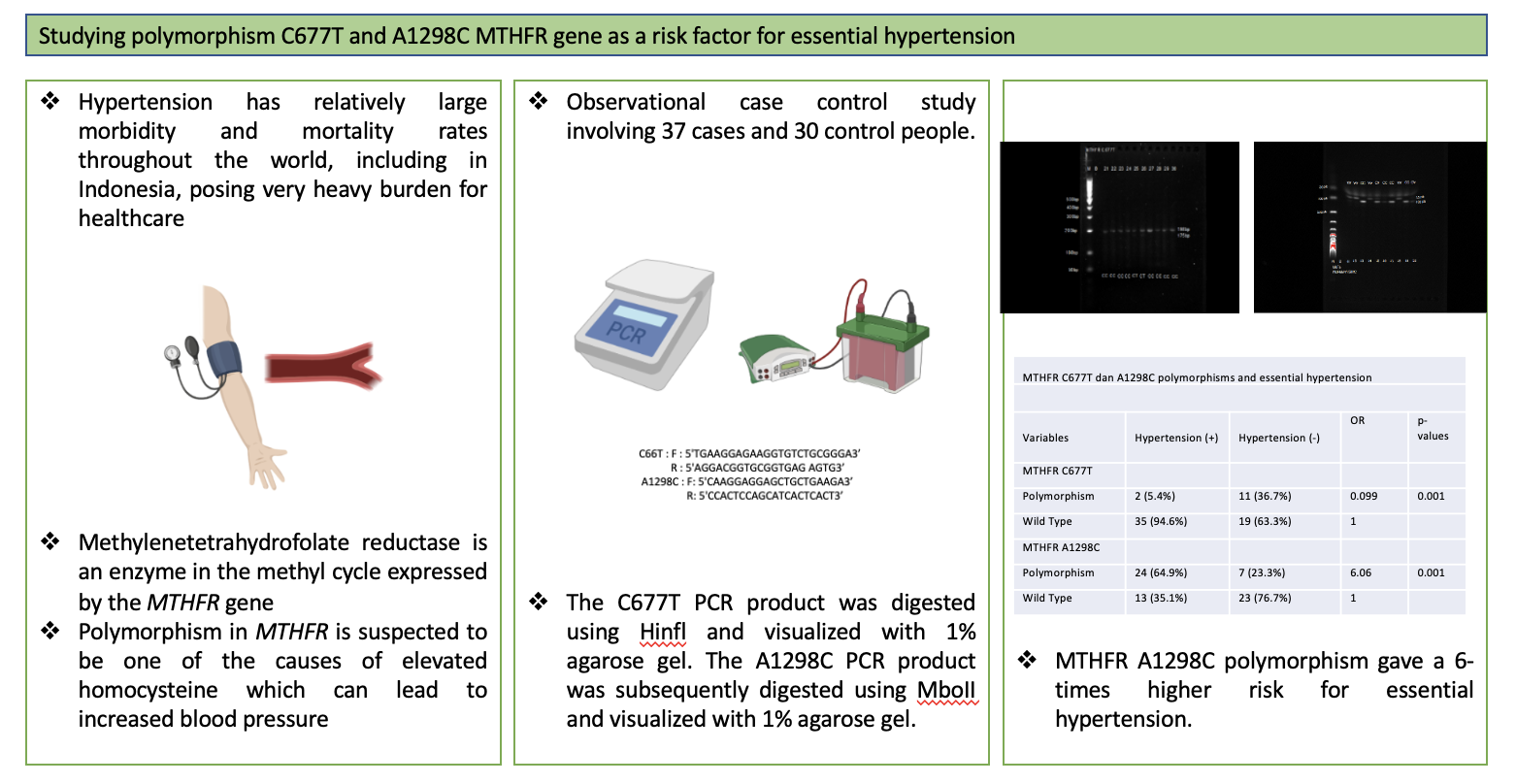
Statistical analysis showed significant relations between MTHFR C6777T with essential hypertension but not a risk factor of essential hypertension (p=0.001, OR=0.099), while MTHFR A1298C showed statistical significance with essential hypertension and also a risk factor of essential hypertension (p=0.001, OR=6.06) (Table 3).
Table 1 Demographic
data of participants
|
Characteristics |
Case (n = 37) |
Control (n = 30) |
p-values |
|
Age |
|
|
|
|
At collection |
40.30 |
40.50 |
0.070 |
|
Sex |
|
|
|
|
Men |
22 (59.5%) |
18 (60%) |
|
|
Women |
15 (40.5%) |
12 (40%) |
|
There is no significant difference in the average age between the case and control groups with similar percentages between male and female subjects in both groups.
Table 2
Allelic and genotype distribution for MTHFR C677T dan A1298C
|
Variables |
Essential Hypertension (+) |
Essential Hypertension (-) |
|
MTHFR C677T |
|
|
|
genotype |
|
|
|
CC |
35 (94.6%) |
19 (63.4%) |
|
CT |
1 (2.7%) |
10 (33.3%) |
|
TT |
1 (2.7%) |
1 (3.3) |
|
Allele |
|
|
|
C |
71 (95.9%) |
48 (80%) |
|
T |
3 (4.1%) |
12 (20%) |
|
MTHFR A1298C |
|
|
|
genotype |
|
|
|
AA |
13 (35.1%) |
23 (76.7%) |
|
AC |
17 (45.9%) |
4 (13.3%) |
|
CC |
7 (19%) |
3 (10%) |
|
Allele |
|
|
|
A |
43 (58.1%) |
50 (83.5%) |
|
C |
31 (41.9%) |
10 (16.7%) |
Table 3 MTHFR C677T dan A1298C
polymorphisms and essential hypertension
|
Variables |
Hypertension (+) |
Hypertension (-) |
OR |
p-values |
|
MTHFR C677T |
|
|
|
|
|
Polymorphism |
2 (5.4%) |
11 (36.7%) |
0.099 |
0.001 |
|
Wild Type |
35 (94.6%) |
19 (63.3%) |
1 |
|
|
MTHFR A1298C |
|
|
|
|
|
Polymorphism |
24 (64.9%) |
7 (23.3%) |
6.06 |
0.001 |
|
Wild Type |
13 (35.1%) |
23 (76.7%) |
1 |
|
In the case group, 64.9% subjects with
A1298C MTHFR polymorphism, with only 23.3% in the control group showing a
statistically significant relation between A1298C polymorphism and a risk
factor for essential hypertension with an odds ratio six times higher than
subjects without the polymorphism (p=0.001). In contrast, only two subjects
(5.4%) in the case group have the C677T MTHFR polymorphism compared to 11
subjects (36.7%) in the control group with odds ratio <1, meaning that the
polymorphism is not statistically significant as a risk factor for essential
hypertension.
This result is different compared to the previous MTHFR C677T research by Candrasarta in 2010 on 213 patients and 202 controls in Indonesia. The research showed a statistical significance p-value of 0.001 and OR of 2.1. The difference in results can probably be attributed to the sample size that made this research statistically significant but not as a risk factor (Candrasatria et al., 2020). The result in this study is similar to previous studies in MTHFR C677T and A1298C in mothers having children with Down syndrome, where the result is not statistically significant for C677T polymorphism but statistically significant for A1298C (Pratamawati et al., 2018). Other research regarding MTHFR A1298C in Indonesian rheumatoid heart disease showed significant results (Nauphar et al., 2019). The results of this study are also in line with the research of Rochmah et al. (2018), there was a significant relationship between MTHFR A1298C and no relationship with C677T in non-syndromic cleft lips/palate cases in the Indonesian Sasak tribe (Rochmah et al., 2018).
Table 4 MTHFR C677T polymorphisms
in different populations
|
First Author |
Year |
Country |
Ethnicity |
Diagnostic |
genotype | |||
|
Case |
Control |
P value |
| |||||
|
Fridman (Fridman
et al.,
2013) |
2013 |
Argentina |
White |
SBP >140 DBP > 90 |
CC: 29 CT: 40 TT: 6 |
CC: 71 CT: 64 TT: 15 |
0.917 |
|
|
Yin (Yin et al., 2012) |
2012 |
China |
Asian |
SBP >140 DBP > 90 |
CC: 244 CT: 358 TT: 68 |
CC: 322 CT: 309 TT: 51 |
0.047 |
|
|
|
|
|
|
|
|
|
|
|
|
Nakata (Nakata et al., 1998) |
1998 |
Japan |
Asian |
SBP >160 DBP > 95 |
CC: 63 CT: 91 TT: 19 |
CC: 65 CT: 83 TT: 36 |
0.309 |
|
|
|
|
|
|
|
|
|
|
|
|
Deshmukh (Deshmukh et al., 2009) |
2009 |
United States |
White |
SBP >140 DBP > 90 |
CC: 22 CT: 16 TT: 4 |
CC: 52 CT: 48 TT: 28 |
0.221 |
|
Abbreviations: SBP: Systolic blood pressure; DBP: Diastolic
blood pressure
We can see the difference between the
C667T polymorphisms MTHFR gene in hypertension in different populations, while
MTHFR A1298C polymorphism studies are still rarely done in hypertension.
MTHFR C677T polymorphism is statistically
significant with essential hypertension but is not a risk factor in essential
hypertension. In contrast, MTHFR A1298C is also statistically significant
inessential hypertension and is a risk factor in essential hypertension.
Individuals with A1298C polymorphism have a six times increased risk of
developing essential hypertension. Findings from this research can be used for
further research, such as haplotype analysis or downstream analysis with
next-generation sequencing for the MTHFR gene.
We thank Beben Benyamin for his valuable discussions
and helpful comments. The Faculty of Medicine Universitas Swadaya Gunung Jati
Internal Research Fund 2021 fully funds this research.
Bell, K., Twiggs, J., Olin, B.R., 2015. Hypertension: The Silent Killer:
Updated JNC-8 Guideline Recommendations. Alabama Pharmacy Association,
Volume 2015, p. 4222
Candrasatria, R.M., Adiarto, S., Sukmawan, R., 2020.
Methylenetetrahydrofolate Reductase C677T Gene Polymorphism as a Risk Factor
for Hypertension in a Rural Population. International
Journal of Hypertension, Volume 2020, p. 4267246
Carlus SJ, Abdallah AM, Bhaskar LV, Morsy MM, Al-Harbi GS, Al-Mazroea AH,
Al-Harbi KM. 2016. The MTHFR C677T polymorphism is associated with mitral valve
rheumatic heart disease. Eur Rev Med Pharmacol Sci. Volume 20(1), pp. 109-14.
Choudhury, I., Jothimalar, R., Patra, A.K., 2012. Angiotensin Converting
Enzyme Gene Polymorphism and Its Association with Hypertension in South Indian Population. Indian Journal of Clinical Biochemistry,
Volume 27(3), pp. 265–269
Cortese, C., Motti, C., 2001. MTHFR Gene Polymorphism, Homocysteine and
Cardiovascular Disease. Public Health Nutrition, Volume 4(2b), pp.
493–497
Deshmukh, A. Rodrigue, K.M., Kennedy, K.M., Land, S., Jacobs, B.S., Raz,
N., 2009. Synergistic Effects of The MTHFR C677T Polymorphism and Hypertension
on Spatial Navigation. Biological Psychology, Volume 80(2), pp. 240–245
Dhanachandra-Singh, K., Jajodia, A., Kaur, H., Kukreti, R., Karthikeyan,
M., 2014. Gender Specific Association of RAS Gene Polymorphism with Essential
Hypertension: A Case-Control Study. BioMed Research International,
Volume 2014, p. 538053
Evangelou, E., Warren, H.R., Mosen-Ansorena, D., Mifsud, B., Pazoki, R.,
Gao, H, Ntritsos, G., Dimou, N., Cabrera, C.P., Karaman, I., Ng, F.L.,
Evangelou, M., Witkowska, K., Tzanis, E., Hellwege, J.N., Giri, A., Edwards,
D.R.V., Sun, Y.V., Cho, K., Gaziano,
J.M., Wilson, P.W.F., Tsao, P.S., Kovesdy, C.P., Esko, T., the Million Veteran
Program, 2018. Genetic Analysis of Over
1 million People Identifies 535 New Loci Associated With Blood Pressure
Traits’, Nature Genetics, Volume 50(10), pp. 1412–1425
Fridman, O. Porcile, R., Morales, A.V., Gariglio, L.O., Potenzoni, M.A.,
Turk Noceto, P.C., 2013. Association of Methylenetetrahydrofolate Reductase
Gene 677C>T Polymorphism with Hypertension in Older Women in a Population of
Buenos Aires City. Clinical and Experimental Hypertension, Volume 35(3),
pp. 159–166
Huang, T., Shu, Y., Cai, Y.D., 2015. Genetic Differences Among Ethnic
Groups. BMC Genomics, Volume 16(1), pp. 1–10
Kedar, R., Chandel, D., 2019. MTHFR Gene Polymorphism and Associated
Nutritional Deficiency in The Etiology and Pathogenesis of Down Syndrome.
Egyptian Journal of Medical Human Genetics, Volume 20(1), pp. 1–10
Indonesian Ministry of Health, 2018. Basic Health Research Results, Volume 53(9), pp. 1689-1699.
P2TPM Ministry of Health, 2019. Hypertension/
high Blood Pressure, P2PTM directorate. Available online at https://p2ptm.kemkes.go.id/infographic/apa-itu-hipertensi-tekanan-darah-tinggi (accessed : 27 November 2022)
Leclerc, D., Sibani, S., Rozen, R., 2013. Molecular Biology of
Methylenetetrahydrofolate Reductase (MTHFR) and Overview of
Mutations/Polymorphisms. In: Madame Curie Bioscience Database, Landes
Bioscience
Nakata, Y., Katsuya, T., Takami, S., Sato, N., Fu, Y., Ishikawa, K.,
1998. Methylenetetrahydrofolate Reductase Gene Polymorphism: Relation to Blood
Pressure and Cerebrovascular Disease. American Journal of Hypertension, Volume 11 (8), pp. 1019–1023
Nauphar, D., Pratamawati, T.M., Sanif, M.E., 2019. P3132
Methylenetetrahydrofolate Reductase A1298C as a Risk Factor for Rheumatic Heart
Disease in Indonesian Population. European Heart Journal, Volume 40, p.
ehz745.0207
P2TPM Ministry of Health, 2019. Types of Stroke, P2PTM directorate. Available
online at https://p2ptm.kemkes.go.id/infographic-p2ptm/stroke/jenis-jenis-stroke (accessed : 27 November 2022)
Padmanabhan, S., Caulfield, M., Dominiczak, A.F., 2015. Genetic and
Molecular Aspects of Hypertension. Circulation Research, Volume 116(6),
pp. 937–959
Pratamawati, T.M. Winarni T.I., Hardian, Faradz, S.M.H., 2018. Maternal
MTHFR A1298C not C677T Polimorphism as the Risk Factor in Children with Down
Syndrome. Research Journal of Medical Sciences, Volume 12(6), pp. 84–89
Rochmah, Y.S., Suwarsi L, Harumsari, S., Sosiawan, A., Fatimah-Muis, S.,
Faradz, S. M., 2018. Maternal Polymorphism MTHFR A1298C not C677T and MSX1 as
the Risk Factors of Non-syndromic Cleft Lips /Palate in Sasak Tribe Indonesia.
Journal of Dental and Medical Research, Volume 11(1), pp. 120–123
Shi, J., Liu, S., Guo, Y., Liu, S., Xu, J., Pan, L., Hu, Y., Liu, Y.,
Cheng, Y., 2021. Association Between eNOS rs1799983 Polymorphism and
Hypertension: a Meta-Analysis Involving 14,185 Cases and 13,407 Controls. BMC
Cardiovascular Disorders, Volume 21(1), pp. 1–11
Singh, S., Shankar, R., Singh, G.P., 2017. Prevalence and Associated Risk
Factors of Hypertension: A Cross-Sectional Study in Urban Varanasi. International
Journal of Hypertension, Volume 2017. p. 5491838.
Stover, P.J., MacFarlane, A.J., Field, M.S., 2015. Bringing Clarity to
the Role of MTHFR Variants in Neural Tube Defect Prevention. American
Journal of Clinical Nutrition, Volume 101(6), pp. 1111–1112
Unger, T., Borghi, C., Charchar, F., Khan, N.A., Poulter, N.R.,
Prabhakaran, D., Ramirez, A., Schlaich, M., Stergiou, G.S., Tomaszewski, M.,
Wainford, R.D., Williams, B., Schutte, A. E., 2020. 2020 International Society
of Hypertension Global Hypertension Practice Guidelines. Hypertension,
Volume 75(6), pp. 1334–1357
Ward, M., Hughes, C.F., Strain, J.J., Reilly, R., Cunningham, C., Molloy,
A.M., Horigan, G., Casey, M., McCarroll, K., O’Kane M., Gibney, M.J., Flynn,
A., Walton, J., McNulty, B.A., McCann, A., Kirwan, L., Scott, J.M., McNulty,
H., 2020. Impact of The Common MTHFR 677C?T Polymorphism on Blood Pressure in Adulthood and
Role of Riboflavin in Modifying The Genetic Risk of Hypertension: Evidence From
the JINGO Project. BMC Medicine, Volume 18(1), pp. 1–11
Wiemels, J.L., Smith, R.N., Taylor, G.M., Eden, O.B., Alexander, F.E.,
Greaves, M.F., United Kingdom Childhood Cancer Study investigators, 2001.
Methylenetetrahydrofolate Reductase (MTHFR) Polymorphisms and Risk of
Molecularly Defined Subtypes of Childhood Acute Leukemia. In:
Proceedings of the National Academy of Sciences, Volume 98(7), pp. 4004–4009
Wilson, C.P., McNulty, H., Ward, M., Strain, J.J., Trouton, T.G., Hoeft,
B.A., Weber, P., Roos, F.F., Horigan, G., McAnena, L., Scott, J.M., 2013. Blood
Pressure in Treated Hypertensive Individuals with the MTHFR 677TT Genotype is
Responsive to Intervention with Riboflavin: Findings of z Targeted Randomized
Trial. Hypertension, Volume 61(6), pp. 1302–1308
Yin, R.X., Wu, J.Z., Liu, W.Y., Wu, D.F., Cao, X.L., Miao, L., Aung,
L.H.H., Zhang, l., Long, X.J., Li, M., Pan, S. L. (2012). Association of
Several Lipid-Related Gene Polymorphisms and Blood Pressure Variation in the
Bai Ku Yao Population. American Journal of Hypertension, Volume 25(8), pp.
927–936
Zaghloul, A., Iorgoveanu, C., Desai, A., Balakumaran, K., Chen, K., 2019.
Methylenetetrahydrofolate Reductase Polymorphism and Premature Coronary Artery
Disease. Cureus, Volume 11(6). p. 5014
