Phytochemical Analysis and Antifungal Activity of Brunei Propolis Against Candida sp. and Cryptococcus sp.
Corresponding email: sahlan@che.ui.ac.id
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6135
Sabrina, G., Adawiyah, R., Usman, A., Mayhana, S.C., Sihotang, D.I.Z., Sahlan, M., 2022. Phytochemical Analysis and Antifungal Activity of Brunei Propolis Against Candida sp. and Cryptococcus sp. International Journal of Technology. Volume 13(8), pp. 1640-1650
| Geani Sabrina | Departement of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, 16424, Indonesia |
| Robiatul Adawiyah | 1. Department of Parasitology, Universitas Indonesia, Kampus UI Salemba, 10430, Indonesia, 2. Master Program in Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Kampus UI Salemba, 104 |
| Anwar Usman | Department of Chemistry, Faculty of Science, Universiti Brunei Darussalam, Gadong BE1410, Brunei |
| Shuffa Chilla Mayhana | Faculty of Medicine, Universitas Indonesia, Kampus UI Salemba, 10430, Indonesia |
| Diandra Imanuella Zilver Sihotang | Faculty of Medicine, Universitas Indonesia, Kampus UI Salemba, 10430, Indonesia |
| Muhamad Sahlan | 1. Departement of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, 16424, Indonesia, 2. Research Center Biomedical Engineering, Faculty of Engineering, Universita |
The propolis used in this study is propolis from Brunei Darussalam,
including G. thoracica, H. itama, and T. binghami. This study focuses on
evaluating phytochemicals, including the total content of polyphenols and
flavonoids, marker compounds, and the anti-fungal activity of propolis Brunei.
Until now, research on the compounds contained in propolis is still being
carried out. However, the literature on the chemical compound of Brunei
propolis is still limited. The results of the research on the content of Brunei
propolis using LC-MS/MS found as many as 21 chemical compounds and three marker
compounds, namely maslinic acid, D-(-) Mannitol, and 18-?-Glycyrrhetinic acid.
The total content of flavonoid and polyphenolic propolis in Brunei was obtained
using quercetin as a flavonoid standard and gallic acid as a polyphenol
standard. In Brunei propolis, the total flavonoid content was higher than the
total polyphenol content. Where the total polyphenol content of propolis G.
thoracica, H. itama, and T. binghami were 78.79±17.06; 70.51±12.93
and 16.37±0.53 (mgGAE/g). while the total flavonoid content was 19.30±1.99;
101.10±6.26 and 61.63±4.53 (mg QE/g). The antifungal activity was carried out
by agar diffusion and microdilution methods. Brunei propolis extract showed
antifungal activity against Cryptococcus and C. Albicans, whereas
propolis Brunei extract showed anti-fungal activity with intermediate
resistance to both fungi.
Anti-fungal; Brunei propolis; LC-MS/MS; Total flavonoid content; Total polyphenol content
Stingless
bees consist of more than 500 species, and it is possible that more than 100
unidentified species, usually stingless bees, can be found in dry and humid
tropical forests and some subtropical areas (Michener,
2012). They
are ecologically active and play an important role in the forest ecosystem.
Stingless bees are also attractive because of their honey, wax, and propolis (Ajibola et al., 2012). Propolis is a natural
resin produced from bees, derived from a mixture of bee saliva containing
enzymes with tree sap or exudates (Pratami et al.,
2020).
The chemical compound content of propolis
itself depends on the plant source, geographical location, environmental
conditions, and bee species (Pratami et al., 2020).
The most common chemical compounds contained in propolis are polyphenols and
flavonoids (Król et al., 2013; Kumar et al.,
2008; Bankova et al., 2000; Hegazi et al., 2000).
Propolis is widely used in traditional medicine, especially in people who have
inadequate health and sanitation conditions (Sung
et al., 2017; Veiga et al., 2017; Boukraâ et al., 2009; Trusheva et al., 2007; Uzel et al., 2005).
Fungal infections are still a health
problem in Indonesia, with 5.3 million people suffering from fungal infections
every year. In fact, the National Nosocomial Infections Surveillance System
(NNISS) reports that Candida species are the fourth most common nosocomial
bloodstream pathogen (Wisplinghoff et al., 2004).
Mortality is estimated at 45% (Cheng et al., 2005),
probably due to ineffective diagnostic methods and inadequate initial
antifungal therapies (Morrell et al., 2005).
Many medical fungi in circulation have undesirable or highly toxic side effects
(amphotericin B), produce relapse, and indicate drug-drug interactions
(azoles). Leading to the development of resistance (fluconazole,
5-flucytosine); some even show ineffectiveness (White
et al., 1998).
Therefore, it is necessary to find and
find new antifungal agents that are more effective and less toxic in overcoming
these problems. Propolis has attracted the attention of scientists searching
for an alternative therapeutic drug against infectious diseases and
multidrug-resistant bacteria since the 1970s. Researchers interest in this
complex substance has increased in recent decades based on further
investigation of the chemical composition of propolis (Toreti
et al., 2013). The use of propolis is very influential on human health
and is used for various purposes. Currently, it is used as an antibacterial,
antifungal, anti-inflammatory, antiviral, anesthetic, and antioxidant (Omar et al., 2017; Boukraâ et al., 2009).
Propolis is found in various regions of
the world, one of which is Brunei Darussalam. However, there are still very few
studies on phytochemicals, compounds contained in Brunei propolis, and
antifungal biological activity against Candida albicans and Cryptococcus
neoformans. Because of the background that underlies this research, it is shown
to analyze the potential of phytochemicals and biological activity contained in
propolis Brunei. This analysis is expected to broaden the spectrum of herbal
medicines that can be used in antifungal treatment.
The Research was conducted with three
different species of propolis because each stingless bee has unique and
specific characteristics. Depending on different countries and regions that may
have unique and specific species of stingless bees, local ecosystemadapts to
this. According to Abdullah et al. (2020) there
are at least 50 species in Kalimantan, including Brunei, Sabak, and Sarawak.
Among them, the species Geniotrigona thoracica, Heterotrigona itama, and
Tetrigona binghami are widely cultivated because their tree trunks are
found in natural forests, are collected, and are easy to cultivate and care for
in suburban areas.
2.1.
Materials
The
sample of propolis extract used came from Brunei Darussalam with three
different species, namely Geniotrigona thoracica, Heterortigona itama, and
Terigona binghami.
Three
species are different in their colour and size. The average body size of Heterotrigona
itama was 4.7 ± 1.55 mm whilst Geniotrigona thoracica was 7.44 ±
2.05 mm, Tetrigona binghami has the permanent space covering 2/3 of the
size of the head. Heterotrigona itama are black in color with grey wings, while
on the contrary, Geniotrigona thoracica are brown in color with dark
brown wings and white tips at the apex of the wings; Tetrigona binghami the
wing color is black except for having a white patch at the tip of the wing (Azmi et al., 2019).
2.2. Identification of Propolis Brunei Content
2.2.1. Total Polyphenol Content (TPC)
Quantitative
testing of polyphenol content was carried out using Feline Ciocalteu and Na2CO3
reagents. 50 mg of gallic acid was used as a standard, dissolved in methanol to
a concentration of 1000 ppm, and then diluted with water to concentrations of
0, 12.5, 25, 37.5, 50, 62.5, 75, 87.5, 100, and 112.5 ppm. Every 0.5 mL of
gallic acid with a different concentration of 0.5 mL of propolis sample was
mixed into 5 ml of Folin reagent, then vortexed and allowed to stand for 5
minutes. Next, 4 mL of 1M Na2CO3 was added to the mixture
and allowed to stand at room temperature for 15 minutes. Using a UV-VIS
spectrometer, the resulting mixture was then measured at a wavelength of 752
nm. Measurements were carried out 3 times (Sahlan
et al., 2020).
2.2.2. Total Flavonoid Content (TPC)
Quantitative
testing of flavonoid content was carried out using standard quercetin as much
as 10 mg dissolved in methanol to reach a concentration of 1000 ppm which was
then diluted with water to concentrations of 0, 12.5, 25, 37.5, 50, and 62.5
ppm. 1 mL of propolis sample was put into a test tube, and 0.2 mL of 10% AlCL3,
0.2 ml of KCH3COO, 5.6 mL of water and 3 mL of methanol were added.
Then the solution was vortexed and allowed to stand at room temperature for 30
minutes. The resulting mixture was then measured at a wavelength of 428 nm
using a UV-VIS spectrometer. Measurements were carried out three times (Sahlan et al., 2020).
2.2.3. LC-MS/MS Test
In
this study, the propolis compound was identified using LC-MS/MS UHPLC Vanquish
Tandem Q Exactive Plus Orbitrap HRMS ThermoScientific in Advanced Research
Laboratory IPB University Bogor, West Java. The eluent phase is formic acid and
acetonitrile with a flowrate 0.2 mL/min for 30 minutes. Mass spectrum data was
analyzed using Compound Discover 3.2 (Pratami et
al., 2018).
2.3. Antifungal Activity
2.3.1 Propolis Preparation
Preparation
of propolis samples for use as a test material includes dilution of propolis in
various concentrations. The concentrations to be tested are 50%, 70% and 100%.
This propolis preparation was carried out with the addition of DMSO which would
later act as a positive control in the antifungal test.
2.3.2 Agar Diffusion Method
The
principle of the agar diffusion method is to make several holes in the Mueller
Hinton agar which has been planted with fungi, namely Cryptococcus
neoformans and Candida albicans. First for three concentrations of
Brunei propolis (50%, 70% and 100%), two for positive controls (K+) including
amphotericin B and fluconazole, and the last for DMSO as a negative control
(K-). Then the inhibition zone will be seen. showing the sensitivity response
of Cryptococcus and C. Albicans to Brunei propolis extract and for
positive-negative controls.
The
first procedure was to test the antifungal activity, sterilize distilled water,
and then pour into a test tube. In the second stage, remove the fungal culture
(Candida albicans and/cryptococcus) with Ose and put it in a test
tube containing sterile distilled water until it reaches 0.5 McFarland. In the
third stage, spread the suspension of fungi (Candida albicans and/cryptococcus)
on the Müeller Hinton agar evenly using a spreader. The fourth stage is wetting
the blank disc with three concentrations of Brunei propolis. The fifth step is
to prepare a 5mg/ml Amphotericin B and 5mg/ml Fluconazole solution. The sixth
step is to take a plain disc that has been soaked in sterile Brunei Propolis
and Aquadest and a 5mg/ml solution of Amphotericin B disc with tweezers and
place it on Mueller Hinton agar which has been smeared with Candida albicans
suspension. The seventh stage, incubating at 37oC for 24 hours in an
incubator (Apriyanto, 2002). The eighth
stage measures the inhibition’s diameter using a caliper from the back of the
petri dish. in the ninth stages, the petri dish must be placed on a dark base
with a flat surface. Then the diameter to be measured includes the diameter of
the disc and is measured by the point closest to the emergence of microbes (Marston, 2011).
3.1. Propolis Brunei Content
This result will show
quantitative analysis of the total content of polyphenols and flavonoids along
with a qualitative analysis of LC-MS/MS to identify the compounds of Brunei Propolis.
3.1.1. Total Content of Polyphenols and Flavonoids
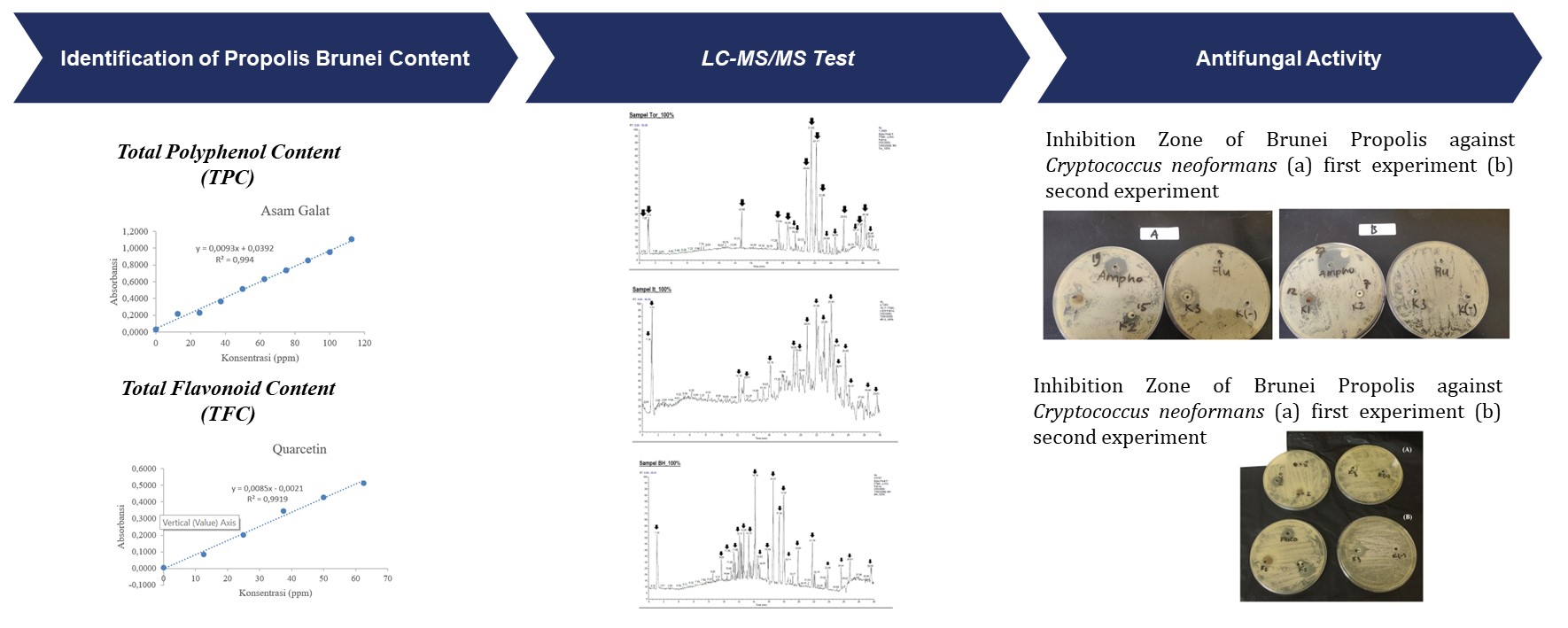
The samples used in the polyphenol and flavonoid content test came from Brunei with three different species, namely Geniotrigona thoracica (Thor code), Heterotrigona itama (IT code), and Tetrigona binghami (Bh code). In measuring the gallic acid calibration curve, the as you can see in Figure 1 results of the calibration curve equation obtained are y = 0.0093x + 0.0392 with a value of R2 = 0.994. With the sample absorbance data, the calculation is carried out using the calibration curve equation (Table 2). Furthermore, the conversion was carried out so that the total polyphenol content.
Figure 1 Standard Curve of Total
Polyphenol Content
Table 1 Calculation of Total Polyphenol
Content
|
m sample (g) |
Sample Code |
Absorbance |
Concentration (ppm) |
Concentration (mgGAE/g) |
Everage Concentra-tion |
Standard Deviation |
TPC (mgGAE/g) |
|
0,432 |
Tor |
0,7720 |
78,7957 |
91,1987 |
78,79 |
17,06 |
78,79±17,06 |
|
0,7290 |
74,1720 |
85,8473 | |||||
|
0,5160 |
51,2688 |
59,3389 | |||||
|
0,437 |
IT |
0,4910 |
48,5806 |
55,5843 |
70,51 |
12,93 |
70,51±12,93 |
|
0,6730 |
68,1505 |
77,9754 | |||||
|
0,6730 |
68,1505 |
77,9754 | |||||
|
0,442 |
BH |
0,1780 |
14,9247 |
16,8832 |
16,52 |
0,53 |
16,37±0,53 |
|
0,1770 |
14,8172 |
16,7615 | |||||
|
0,1700 |
14,0645 |
15,9101 |
(TPC) of EEP was 100% of each propolis species. Propolis from G. thoracica species had the highest polyphenol content, namely 78.79±17.06 mgGAE/g propolis. At the same time, the lowest polyphenol content value of 16.37±0.53 mgGAE/g propolis was owned by propolis from T. binghami species.
Figure 2 Standard Curve of Total Flavonoid Content
Meanwhile, in the test
for the Total Flavonoid Content, the quercetin calibration curve was the
measure (Figure 2) with the results of the calibration curve equation being y =
0.0085x - 0.0021 and the value of R² = 0.9919. Propolis from H. itama
species had the highest flavonoid content value, 101.10±6.26 mg QE/g propolis.
Meanwhile, the species with the lowest total flavonoid content was G.
thoracica at 19.30±1.99 mg QE/g propolis (Table 2).
Table 2 Calculation of Total Flavonoid
Content
|
m sample (g) |
Sample Code |
Absorbance |
Concentration (ppm) |
Concentration (mgGAE/g) |
Everage Concentra- tion |
Standard Deviation |
TFC
(mgGAE/g) |
|
0,432 |
Tor |
0,142 |
16,9529 |
19,6215 |
19,30 |
1,99 |
19,30±1,99 |
|
0,124 |
14,8353 |
17,1705 | |||||
|
0,153 |
18,2471 |
21,1193 | |||||
|
0,437 |
IT |
0,76 |
89,6588 |
102,5845 |
101,10 |
6,26 |
101,10±6,26 |
|
0,698 |
82,3647 |
94,2388 | |||||
|
0,789 |
93,0706 |
106,4881 | |||||
|
0,442 |
BH |
0,498 |
58,8353 |
66,5558 |
61,63 |
4,53 |
61,63±4,53 |
|
0,454 |
53,6588 |
60,7000 | |||||
|
0,431 |
50,9529 |
57,6391 |
3.1.2. Analysis of LC-MS/MS Test Results
In
this study, LC-MS/MS was used to identify the compounds present in 3 species of
Brunei propolis. Identification of the compound was
carried out in two parts. First, identify the highest peak of each propolis
sample and identify the presence of the same compound in propolis. Meanwhile,
the presence of the same compound in propolis was identified to search for
marker compounds from Brunei propolis.
Spectra with peaks at
different retention times were obtained by negative ionization mode. The peaks
in the spectra can be formed due to the presence of an identified compound. In
this study, the signal read on the LC-MS/MS tool was analyzed using the Thermo
Scientific Xcalibur 4.2 software so that the chromatogram. Types of
chromatograms are generally divided into Total Ion Chromatogram (TIC) and Base
Peak Intensity (BPI).
The chromatogram on
the TIC is made based on the sum of all ion currents in the mass spectra series
as a function of retention time. Meanwhile, the BPI chromatogram is obtained by
representing the base peak signal, which shows the highest ion intensity
detected from each mass spectrum as a function of retention time (Murray et al., 2013).
Based on the results
of compound readings (Fig 3.) using Compound Discover 3.2 software in Brunei
propolis samples with Thor code (G. thoracica), 76 compounds were
identified from the database. The identified compounds were obtained as many as
18 highest peaks or 18 compounds with the highest content in propolis Brunei G.
thoracica. Meanwhile, in the H. itama sample, 133 compounds were
identified from the database. List of compounds identified from the 17 highest
peaks or 17 compounds with the most content in H. itama propolis. Then
the sample of T. Binghami, obtained 91 compounds identified from the
database. List of compounds identified from the 20 highest peaks or 20
compounds with the most content in T. Binghami propolis.
Figure 3 LC-MS/MS
Spectra of Brunei Propolis (a) G. thoracica, (b) H. itama, (c) T.
binghami
In the second part,
namely the identification of the presence of the same compound in propolis, the
priority or criteria that can be used to determine the identification of
propolis compounds are carried out. The compound identification process at this
stage is carried out by searching the database owned by PubChem and ChemSpider.
The results obtained in the first section were then processed into the second
section, which obtained active compounds in each species of Brunei propolis,
among others, from 18 compounds with the highest composition in Brunei propolis
G. thoracica, there were four compounds identified. Then, from 17
compounds with the highest composition in Brunei H. itama propolis,
there 10 compounds were identified. In Brunei T. Binghami propolis from
20 compounds with the most composition, there were seven compounds identified.
Table 3 Identification
of propolis compounds using LCMS/MS
|
No |
Compound Name |
Molecular Formula |
Propolis Code |
Group |
|
1 |
Mangostin |
C24H26O6 |
Thor |
Fenol |
|
2 |
Maslinic
Acid |
C30H48O4 |
Triterpenoid | |
|
3 |
Luteolin |
C15H10O6 |
Flavonoid | |
|
4 |
D-(-)Mannitol |
C6H14O6 |
Alcohol
and sugar | |
|
5 |
18-?-Glycyrrhetinic acid |
C30H46O4 |
It |
Glycyrrhizic acid |
|
6 |
Maslinic
Acid |
C30H48O4 |
Triterpenoid | |
|
7 |
Asiatic acid |
C30H48O5 |
Pentacyclic triterpenoid | |
|
8 |
D-(-)Mannitol |
C6H14O6 |
Alcohol
and sugar | |
|
9 |
Glyceryl 2-linolenate |
C21H36O4 |
Fatty Acid | |
|
10 |
(R)-Naproxen |
C14H14O3 |
Naphthalene | |
|
11 |
Flavesone |
C14H20O4 |
Fenol | |
|
12 |
Apigenin |
C15H10O5 |
Flavonoid | |
|
13 |
Ursolic acid |
C30H48O3 |
Fenol | |
|
14 |
10,16-Dihydroxyhexadecanoic
acid |
C16H32O4 |
Bh |
Hydroxy
fatty acid |
|
15 |
(3aR,4R,5R,6aS)-5-Hydroxy-4-[(1E,3S)-3-hydroxy-1-octen-1-yl]hexahydro-2H-cyclopenta[b]furan-2-one |
C15H24O4 |
Aliphatic alcohol | |
|
16 |
Amiloxate |
C15H20O3 |
Cinnamic
acid | |
|
17 |
Azelaic acid |
C9H16O4 |
Fenol | |
|
18 |
Oryzarol |
C26H42O3 |
Benzoate
ester | |
|
19 |
18-?-Glycyrrhetinic acid |
C30H46O4 |
Glycyrrhizic acid | |
|
20 |
D-(-)Mannitol |
C6H14O6 |
Alcohol
and sugar | |
|
21 |
Maslinic Acid |
C30H48O4 |
Triterpenoid |
After identifying the compounds
from the highest peak, three compounds were found in the majority of Brunei
propolis. This shows that the three compounds can be marker compounds for
Brunei propolis. The list of possible marker compounds includes Maslinic acid,
D-(-)-Mannitol, and 18-?-Glycyrrhetinic acid.
From
the results of the LC-MS/MS test on the three Brunei propolis species, several
active chemical compounds were obtained, each of which has various benefits. It
can be seen from the explanation above therefore, the three species of Brunei
propolis have the potential to have benefits as antimicrobial, antioxidant,
anti-inflammatory, and antifungal agents (Rasul
et al., 2013).
3.2. Antifungal Activity
The
antifungal test with propolis was carried out using the agar diffusion method,
where agar diffusion is a diffusion method used to determine the activity of
antifungal agents. Fungal resistance to a radius of 3 mm below the control
diameter, intermediate when the inhibition zone has a radius ? 2 mm and is >
3 mm under control, while resistance is when the diameter of the inhibition has
a radius of < 2 mm. Tables 4 and 5 show the results of the inhibition
diameter measurements for both types of fungi with Brunei propolis.
Table
4 and Figure 4 shows the diameter of the antifungal test inhibition against Cryptococcus
neoformans. The data was carried out twice to see a better level of
accuracy. These data indicate that the diameter of inhibition resulting from
the antifungal test using Brunei propolis indicates that Brunei propolis at
concentrations of 50% and 70% has the moderate inhibitory ability as antifungal
against Cryptococcus neoformans and this fungus has intermediate
resistance to Brunei propolis.
Figure 4 Inhibition Zone of Brunei
Propolis against Cryptococcus neoformans (a) first experiment (b) second experiment
Table 4 Inhibition Zone of Brunei Propolis against Cryptococcus
neoformans
|
A (mm) |
B (mm) |
Average | |||
|
Propolis
50% |
K1 |
0 |
K1 |
12 |
6 |
|
Propolis
70% |
K2 |
15 |
K2 |
7 |
11 |
|
Propolis
100% |
K3 |
0 |
K3 |
0 |
0 |
|
DMSO
10% |
K
(-) |
0 |
K
(-) |
0 |
0 |
|
Amphotericin
B |
K
(+) |
19 |
K
(+) |
27 |
23 |
|
Fluconazole |
K
(+) |
7 |
K
(+) |
0 |
0.5 |
Table 5 and Figure 5
shows the diameter data of the antifungal test inhibitors against Candida
albicans. The resulting inhibition diameter values indicate that Candida
albicans have intermediate resistance to Brunei propolis, and Brunei
propolis has moderate antifungal ability against candida albicans. And
the Brunei propolis inhibition zone with a concentration of 100% greater than
fluconazole indicates that Brunei propolis has the potential to be a better
antifungal agent.
Table 5 Inhibition Zone of
Brunei Propolis against Candida albicans
|
A (mm) |
B (mm) |
Average | |||
|
Propolis
50% |
K1 |
9 |
K1 |
11 |
10 |
|
Propolis
70% |
K2 |
7 |
K2 |
11 |
9.5 |
|
Propolis
100% |
K3 |
10 |
K3 |
13 |
11.5 |
|
DMSO
10% |
K
(-) |
0 |
K
(-) |
0 |
0 |
|
Fluconazole |
K
(+) |
5 |
K
(+) |
16 |
10.5 |
Figure 5 Inhibition Zone of
Brunei Propolis against Candida albicans (a) first experiment (b) second
experiment
The total flavonoid and polyphenol content
test result in each Brunei propolis species showed that H. itama had the
highest total flavolonid content of 61.63±4.53 mg QE/g propolis. Meanwhile, G.
thoracica had the highest total polyphenol content of 78.79±17.06 mgGAE/g
propolis. For the analysis of the identification of propolis compounds using
LC-MS/MS, a total of 21 active chemical compounds and three marker compounds
were found. The
antifungal test results using the agar diffusion method showed that Brunei
propolis had a larger inhibition zone for candida albicans than cryptococcus
neoformans, and the resistance of both fungi was in the intermediate
category.
LCMS/MS analysis was performed at the Advanced Research Laboratory
of IPB University, and the Antifungal test was performed at Laboratory of the
Department of Parasitology Faculty of Medicine Universitas Indonesia is
gratefully acknowledged.
Abdullah,
N.A., Zullkiflee, N., Zaini, N.S.Z., Taha, H., Hasmin, F., Usman, A., 2020.
Phytochemicals, Mineral Contents, Antioxidants, and Antimicrobial Activities of
Propolis Produced by Brunei Stingless Bees Geniotrigona Thoracica, Heterotrigona
Itama, And Tetrigona Binghami. Saudi Journal of Biological Sciences,
Volume 27(11), pp. 2902–2911
Ajibola,
A., Chamunorwa, J.P., Erlwanger, K.H., 2012. Nutraceutical Values of Natural
Honey and Its Contribution to Human Health and Wealth. Nutrition &
metabolism, Volume 9(1), pp.1–12
Apriyanto,
D., 2002. Antibacterial Activity of Pepper Powder (Piper nigrum L.) Against
Pathogenic Bacteria and Food Destroyers by Well Method. Thesis. Unila
Agricultural Product Technology Department. Lampung.
Azmi,
W.A., Ghazi, R., Nasharuddin, I.S., 2019. Morphological, Nest Architecture and
Colony Characteristics of Stingless Bees (Hymenoptera; Apidae; Meliponini) from
Tasik Kenyir, Terengganu. In Greater Kenyir Landscapes. Springer, Cham, pp
111–121
Bankova.
V.S., Castro, D.S.L., Marcucci, M.C., 2000. Propolis: Recent Advances in
Chemistry and Plant Origin. Apidologie, Volume 31(1), pp. 3–15
Boukraâ,
L., Sulaiman, S.A., 2009. Rediscovering The Antibiotics of The Hive. Recent
patents on anti-infective drug discovery, Volume 4(3), pp. 206–213
Cheng,
M.F., Yang, Y.L., Yao, T.J., Lin, C.Y., Liu, J.S., Tang, R.B., Yu, K.W., Fan,
Y.H., Hsieh, K.S., Ho, M., Lo, H.J., 2005. Risk Factors for Fatal Candidemia
Caused by Candida Albicans and Non-Albicans Candida Species. BMC
Infectious Diseases, Volume 5(1), pp. 1–5
Hegazi,
A.G., Abd El Hady, F.K., Abd Allah, F.A., 2000. Chemical Composition and
Antimicrobial Activity of European Propolis. Zeitschrift für Naturforschung
C, Volume 55(1-2), pp. 70–75
Król,
W., Bankova, V., Sforcin, J.M., Szliszka, E., Czuba, Z., Kuropatnicki, A.K.,
2013. Propolis: Properties, Application, and Its Potential. Evidence-Based
Complementary and Alternative Medicine, Volume 2013
Kumar,
N., Mueen Ahmad, K.K., Dang, R., Husain, A., 2008. Antioxidant and Antimicrobial
Activity of Propolis from Tamil Nadu Zone. Journal of Medicinal Plants
Research, Volume 2(12), pp. 361–364
Marston,
A., 2011. Thin-layer Chromatography with Biological Detection in Phytochemistry. Journal
of Chromatography A, Volume 1218(19), pp.2676–2683
Michener,
C. D. 2012. The Meliponini. In Pot-Honey, pp. 3–17. doi:10.1007/978-1-4614-4960-7_1 10.1007/978-1-4614-4960-7_1
Morrell,
M., Fraser, V.J., Kollef, M.H., 2005. Delaying the Empiric Treatment of Candida
Bloodstream Infection until Positive Blood Culture Results are Obtained: A
Potential Risk Factor for Hospital Mortality. Antimicrobial Agents and
Chemotherapy, Volume 49(9), pp. 3640–3645
Murray,
K.K., Boyd, R.K., Eberlin, M.N., Langley, G.J., Li, L., Naito, Y., 2013.
Definitions of Terms Relating to Mass Spectrometry (IUPAC Recommendations
2013). Pure and Applied Chemistry, Volume 85(7), pp. 1515-1609
Omar,
R., Igoli, J.O., Zhang, T., Gray, A.I., Ebiloma, G.U., Clements, C.J.,
Fearnley, J., Ebel, R.E., Paget, T., De Koning, H.P., 2017. The Chemical
Characterization of Nigerian Propolis Samples and Their Activity Against
Trypanosoma Brucei. Scientific reports, Volume 7(1), pp. 1–10
Pratami,
D. K., Mun’im, A., Hermansyah, H., Gozan, M., Sahlan, M., 2020.
Microencapsulation Optimization of Propolis Ethanolic Extract from Tetragonula Spp
Using Response Surface Methodology. International Journal of Applied
Pharmaceutics, Volume 12(1)
Pratami,
D. K., Mun’im, A., Sundowo, A., Sahlan, M., 2018. Phytochemical Profile and
Antioxidant Activity of Propolis Ethanolic Extract from Tetragonula Bee. Pharmacognosy
Journal, Volume 10(1)
Rasul,
A., Millimouno, F.M., Ali Eltayb, W., Ali, M., Li, J., Li, X., 2013.
Pinocembrin: A Novel Natural Compound with Versatile Pharmacological and
Biological Activities. BioMed Research International, Volume 2013, pp. 1–9
Sahlan,
M., Mandala, D.K., Pratami, D.K., Adawiyah, R., Wijarnako, A., Lischer, K.,
Fauzi, A., 2020. Exploration of The Antifungal Potential of Indonesian Propolis
from Tetragonula Biroi Bee on Candida Sp. and Cryptococcus Neoformans. Evergreen,
Volume 7, 118–125
Sung,
S.H., Choi, G.H., Lee, N.W., Shin, B.C., 2017. External Use of Propolis for
Oral, Skin, and Genital Diseases: A Systematic Review and Meta-Analysis. Evidence-Based
Complementary and Alternative Medicine, Volume 2017
Toreti,
V.C., Sato, H.H., Pastore, G.M., Park, Y.K., 2013. Recent Progress of Propolis for
Its Biological and Chemical Compositions and Its Botanical Origin. Evidence-based
complementary and alternative medicine, Volume 2013, pp. 1–13
Trusheva,
B., Trunkova, D., Bankova, V., 2007. Different Extraction Methods of
Biologically Active Components from Propolis: A Preliminary Study. Chemistry
Central Journal, Volume 1(1), pp. 1–4
Uzel,
A., Önçag?, Ö., Çog?ulu, D., Gençay, Ö., 2005. Chemical Compositions and
Antimicrobial Activities of Four Different Anatolian Propolis Samples. Microbiological
research, Volume 160(2), pp. 189–195
Veiga,
R., De Mendonça, S., Mendes, P., Paulino, N., Mimica, M., Lagareiro Netto, A.,
Lira, I., López, B.C., Negrão, V., Marcucci, M., 2017. Artepillin C and
Phenolic Compounds Responsible for Antimicrobial and Antioxidant Activity of
Green Propolis and Baccharis Dracunculifolia DC. Journal of Applied
Microbiology, Volume 122(4), pp. 911–920
White,
T.C., Marr, K.A., Bowden, R.A., 1998. Clinical, Cellular, and Molecular Factors
That Contribute to Antifungal Drug Resistance. Clinical microbiology reviews,
Volume 11(2), pp. 382–402
Wisplinghoff,
H., Bischoff, T., Tallent, S.M., Seifert, H., Wenzel, R.P., Edmond, M.B., 2004.
Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from
A Prospective Nationwide Surveillance Study. Clinical Infectious
Diseases, Volume 39(3), pp. 309–317
