Can Cryoprotectant’s modification in Spermatozoa Cryopreservation be an Alternative to Improve Embryo Quality? A Review
Corresponding email: finallysilvia@gmail.com
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6129
Larasati, M.D., Lestari, S.W., Hestiantoro, A., Pangestu, M., 2022. Can Cryoprotectant’s modification in Spermatozoa Cryopreservation be an Alternative to Improve Embryo Quality? A Review. International Journal of Technology. Volume 13(8), pp. 1755-1767
| Manggiasih Dwiayu Larasati | Doctoral Program in Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jl. Salemba Raya No. 6, Jakarta 10430 Indonesia |
| Silvia Werdhy Lestari | Department of Medical Biology, Faculty of Medicine, Universitas Indonesia, Jakarta 10430 Indonesia |
| Andon Hestiantoro | Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Indonesia, Jakarta 10430 Indonesia |
| Mulyoto Pangestu | Education Program in Reproduction and Development, Department of Obstetrics and Gynecology, School of Clinical Sciences, Monash University, Victoria, Australia |
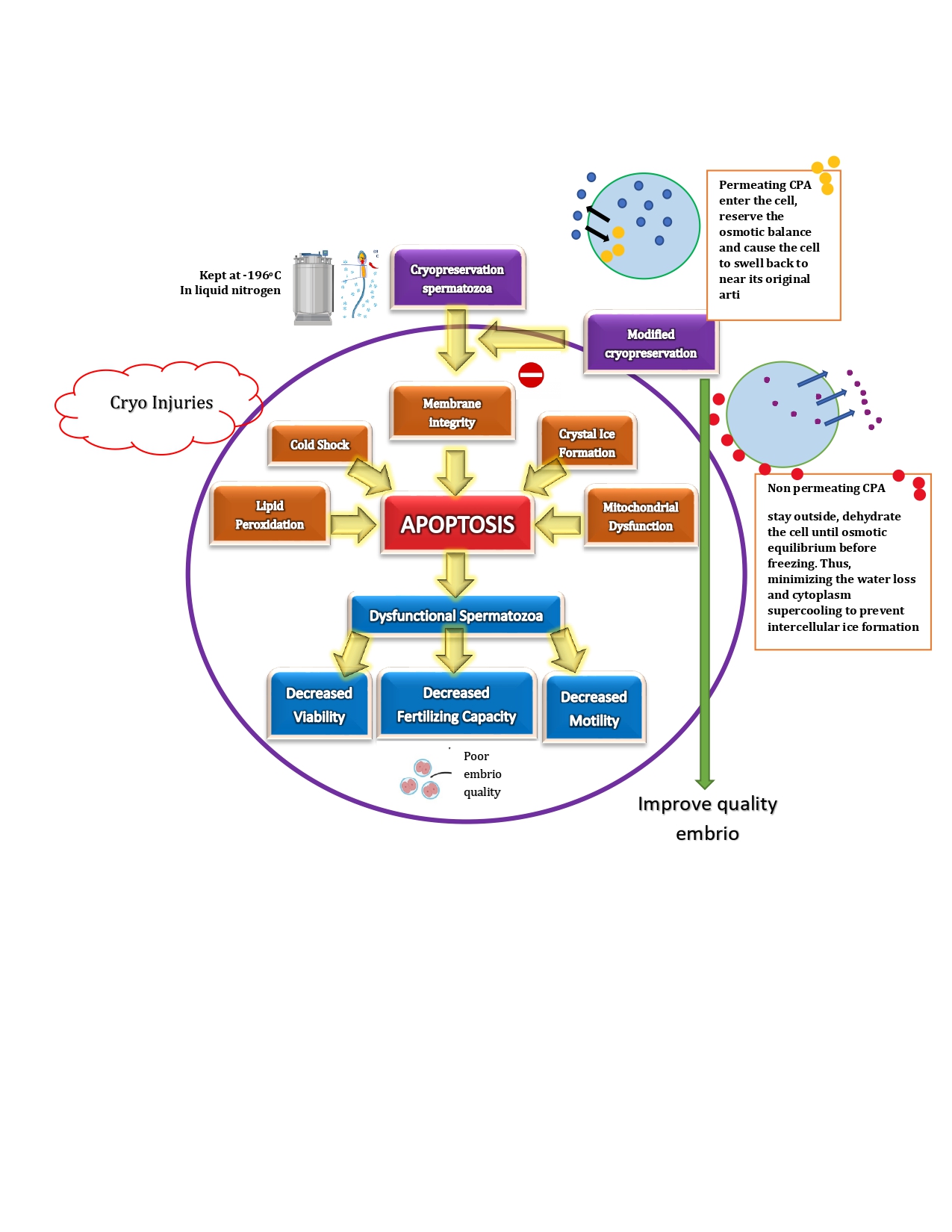
Spermatozoa cryopreservation is an effective method for maintaining male
fertility in humans. Nevertheless, there are some limitations of sperm
cryopreservation, which is called as cell injury by cryoprotectant, that cannot
be avoided. This process will affect embryo quality. Therefore, it is mandatory
to modify cryoprotectant in spermatozoa cryopreservation, to improve embryo
quality. This review aimed to summarize the modification of cryoprotectant that
can damage the cell, thereby improving embryo quality. To this purpose, a
computerized search of EMBASE, PubMed, Scopus and Google Scholar databases from
2008 to 2022 were performed on general term such as “sperm cryopreservation”,
“cryoprotective agent”, “modified cryoprotectant”, “cell injury”. Of these, 1847
publications were screened and 38 articles were obtained and evaluated.
Although no formal conclusions can be drawn regarding the cryopreservation of
spermatozoa to obtain good embryo quality, our results suggest that modified
cryoprotectants can be an alternative cryoprotectant compared to commercial
cryoprotectants. In addition, the use of antioxidant in spermatozoa
cryopreservation can also prevent cell damage due to the negative effects of
cryoprotectants. However, further researches
still need to be performed to investigate the cellular mechanisms.
Cell injury; Cryoprotectant agent; Modified cryoprotectant; Sperm cryopreservation
Cryopreservation is needed for medical purposes such as the preservation of organs, tissues and pharmaceutical research (Bojic et al., 2021). To maintain reproductive ability in women, cryopreservation of adult oocytes is required (Jang et al., 2017). Furthermore, the main reasons for cryopreservation of semen and testicular tissue are autoimmune diseases, neoplasia, spinal cord injury, vasectomy, urological pathology, patients undergoing assisted reproductive programs, or due to changes in spermatogenesis(Gandini et al., 2017) . The first successful embryo cryopreservation dates back in 1996, in a woman with breast cancer diagnosis and undergoing natural IVF before chemotherapy. Overall, cryopreservation is useful for preserving the fertility of men planning to undergo vasectomy or procedures that could compromise their fertility, such as surgery, radiotherapy or chemotherapy (Singer et al., 2019).
Cryopreservation
spermatozoa cells are an effort to store spermatozoa cells in a frozen state so
that they can be used at any time when needed and used in support application
of assisted reproductive technology (Höfner et al., 2020). Cryopreservation is the process of cooling and
storing cells in liquid nitrogen at a temperature of -196oC to stop
all metabolic processes. There are two major methods of cryopreservation - slow
freezing and vitrification (Jang et al., 2017). Slow cooling involves a heating rate of about
1°C/min with a cryoprotectant of less than 1.0 M, using a controlled rate
freezer or a benchtop portable freezing container (Jang
et al., 2017; Li et al. 2019). Slow freezing is advantageous due to the
lower risk of contamination during procedures and requires fewer manipulation
skills.
On
the other hand, slow freezing has a higher risk of extracellular ice formation
that may cause harm to the cells (Agarwal
& Tvrda, 2017; Jang et al., 2017). In recent times, vitrification has become a
more popular alternative to slow freezing. Vitrification is directly changing
the nature of the cell suspension from an aqueous phase to a glassy state with
liquid nitrogen. This process lowers the risk of cell injury and increasing
cell survival rate dramatically. The vitrification technique, however, carries
a higher risk of contamination from pathogenic agents and requires high
manipulation skills (Jang et al., 2017). Slow cooling and vitrification techniques aim to
protect the cells from temperature-induced damage, intracellular ice formation (Sieme et al., 2015), dehydration, and preventing toxic effects at high
and low temperatures (Pegg, 2015). Temperature changes on the process of osmosis
results in an increase in water flux. This situation relates to the effect of
temperature on density and viscosity (Sutijan et al., 2022).
Cryopreservation involves a decrease in
temperature. A further drop in temperature below 0°C causes the water in the
extracellular environment to freeze, resulting in the increase of the
concentration of solutes. The imbalance in solutes creates osmotic pressure,
causing the solvent to flow through the plasma membrane from the inside to the
outside of the cell and the abrupt flow of solvent can affect the structure of
the sperm plasma membrane (Peris-Frau et al., 2020). The effect on the
structural integrity of the membrane threatens the viability of the biological
samples and lowers the quality of cryopreservation. To limit the structural
damage, cryoprotectants that possesses minimum cytotoxic effects are often
utilized (Whaley et al., 2021; Sieme et al., 2016). Unfortunately, the
simple cryoprotective agents still have a negative effect to the cell, related
to cell injury issues. Cell injury is damage that can result from spermatozoa
cryopreservation induced by cellular stress during the cell freezing process.
Cell injury can decrease cell viability due to cold shock and crystal ice
formation (Morris et al., 2012). Direct damage will affect
cellular structure and function, e.g. degradation processes spermatozoa
metabolism, whereas damage does not directly difficult to observe and only seen
after thawing. The main effect of cold shock on spermatozoa cells is a decrease
in motility and vitality, changes in permeability and changes in components
lipids in the spermatozoa membrane. Meanwhile, the effect on spermatozoa cells
is due to the formation of ice crystals is a decrease in motility and viability
spermatozoa, increased release of intracellular enzymes outside the cell, and
damage to organelles such as lysosomes and mitochondria. An optimal freezing
temperature is required before the cells are stored in liquid nitrogen to avoid
cryoinjury. In addition, during cell thawing, cryoprotectants must be removed
to prevent toxicity. Therefore, it is really needed to modify the
cryoprotective agents, so that cells, particularly sperm cells can be preserved
well. This review aimed to summarize the modification of cryoprotectant that
can damage cell injury, thereby improving embryo quality.
This review article was conducted using several search engines such as EMBASE, PubMed and Google Scholar. The relevant publications have been located using a boolean search method (AND, OR, NOT) which included the phrases sperm, vitrification, freezing, cryopreservation, fertility preservation, cryoprotectant and cell injury. The search term ‘sperm cryopreservation’, ‘cryoprotectant agent’, ‘modified cryoprotectant’ and ‘cell injury’ were all included (Figure 1).
Figure 1 Search strategies for literature
review
3.1. Cryoprotectant Agent
Cryoprotectants are substances
nonelectrolyte chemicals that play a role in reducing lethal effect during
freezing either form the influence of the solution and the formation of ice
crystals so that cell viability can be maintained (Whaley et al., 2021; Fahy & Wowk, 2015). Osmotic stress
become dangerous for the cells during frozen storage (Sieme et al., 2015).
Cryoprotectant agents (CPAs) are utilized during the freezing process for tissue
or other biological samples to reduce osmotic stress. Therefore, it is
necessary to identify proper CPAs that are safe for use for biological samples.
Table 1 shows some of the concentrations of cryoprotectants commonly
used during the freezing process. CPAs can be classified into two major
categories: a) cell membrane-permeating cryoprotectants, such as glycerol,
dimethyl sulfoxide (DMSO), ethylene glycol, propylene glycol (Varisli et al., 2009; Sieme et al., 2016) and b) non-membrane permeating cryoprotectants, such as
2-methyl-2,4-pentanediol and polymers such as polyvinyl pyrrolidone,
hydroxyethyl starch, and various sugars. (Whaley et al., 2021; Best, 2015; Sztein
et al., 2001). Based on the
summary of table 1, it is known that glycerol is the best and most commonly
used cryoprotectant for spermatozoa cryopreservation. Because of glycerol has
hydroxyl groups (Ni’mah et al., 2019) so as to
prevent the formation of ice crystals (Hanifah et al., 2020; Hamidi, 2010).
Table 1 A brief
overview of the several forms of CPAs, along with their characteristic
concentrations and properties
|
Cryoprotective
agent |
Commonly
used concentrations |
Organism and biological samples |
Effects and features |
References | |
|
Glycerol |
50% -
70% |
Buffalo
bull sperm, Human
sperm* |
Less hazardous at elevated concentrations |
(Tariq et al. 2020) (Kitporntheranunt et al., 2017) | |
|
DMSO |
5% -
10% |
Sea
bream sperm |
Toxic but widely accessible |
(Zilli et al. 2014) | |
|
Ethylene
glycol |
60% |
Mice
sperm Boar
sperm |
Some toxicity, although the most efficient cryoprotectants |
(Critser & Mobraaten
2000) (Guthrie & Welch 2005) | |
|
Propylene
glycol |
5% -
15% |
Rat
sperm |
A synthetic organic substance that it also utilised as antifreeze |
.(Varisli et al. 2009)
| |
|
Trehalose |
90% |
Ram
semen |
Contains two glucose molecules and is generated by numerous species |
(Pelufo et al. 2015) | |
*GEYC = glycerol egg
yolk citrate.
3.1.1. Glycerol
Glycerol is a typical cryoprotectant that has
been used extensively for storing sperm cells (Sztein et al., 2018; Jang et al., 2017) , and there has been a significant amount of
research on it. Glycerol aids in the protection of cells by crossing the cell
membrane and beginning to influence the water molecules (Best,
2015; Pegg, 2007) . During freezing-induced membrane
phase transitions, glycerol can permeate cellular membranes and affect the rate
and extent of cellular dehydration (Sieme
et al. 2016). Glycerol establishes hydrogen bonds with water molecules, which,
when exposed to freezing temperatures, results in the formation of an amorphous
solid. This process is also referred to as vitrification. After recovery, cells
cryopreserved in glycerol exhibit excellent vitality (Wessel
& Ball, 2004). In addition, glycerol also affects the rate
of reaction and the balance of chemical reactions (Sulistyo
et al., 2020).
3.1.2. Dimethyl sulfoxide
(DMSO)
Similar
to Glycerol, Dimethyl sulfoxide (DMSO) is a permeating agent. It functions
identically to glycerol but is harmful to live cells. Commonly, it is utilised
at a concentration of 10%. Today, it is the most common CPA. DNA methylation
and histone modification have been associated with a decrease in cell survival
and the induction of cell differentiation (Miyagi-Shiohira
et al., 2015).
3.1.3. Ethylene glycol
Additionally,
ethylene glycol is a regularly used CPA. It was discovered that substituting
ethylene glycol for propylene glycol in vitrification solutions lessens the
solution's non-specific toxicity. When combined with water, it modifies the
hydrogen bonding, and water forms the same amorphous solid. Ethylene glycol
makes weaker hydrogen bonds than propylene glycol, therefore macromolecules are
shielded by a greater number of residual water molecules, resulting in
increased hydration. (Bojic
et al., 2021). However, since it cannot pass through the
cell membrane, it is a non-permeable agent that operates on the cell's outside.
3.1.4. Propylene glycol
Propylene
glycol is a non-permeable substance that acts on the cell's outside. It showed
the highest percentage of motility recovery following freezing and warming of
all CPAs examined (about 70%; P <0.05). In addition to that, it is a widely
utilized CPA (Varisli
et al., 2009)
3.1.5. Trehalose
Trehalose
is a kind of sugar that may be produced by various species, including fungi,
bacteria, yeast, and even certain insects and plants (Whaley
et al., 2021). It aids these organisms in their ability to
endure frigid conditions. The optimal method for preparing ram ejaculates for
deep freezing appears to be the simultaneous addition of glycerol and
disaccharide after cooling to 5 °C, utilising trehalose as the impermeable
sugar. Sperm motility after thawing suggested that trehalose possessed a
stronger ability for cryopreservation than sucrose. Consequently, it is applied
in cryopreservation (Pelufo
et al., 2015).
3.2. In Vitro Fertilization and Embryo Quality
Male
fertility is influenced by sperm morphology, sperm motility, plasma membrane
integrity and acrosomal reactions. Further analysis of other factors is needed
to provide a complete picture of the potential for male fertility. In vitro
fertilization process is influenced by sperm and oocyte quality. Many factors
affect sperm quality in freezing and thawing e.g. the freezing method,
temperature control, sperm preparation technique, and type of cryopreservative
agent (Kitporntheranunt
et al., 2017). Likewise, many factors affect oocyte
quality. Ideally, good quality sperm and oocytes will produce good embryos too.
But on the contrary, the embryo quality will decrease if the sperm quality is
low due to frozen storage. Table 2 describes the research results
related to in vitro fertilization using sperm cryopreservation. Embryo quality
can be measured through the normal stages of cell division and blastocyst. On
day three, embryos of high grade are identified as having 7-8 uniformly sized
cells that are not fragmented (Lestari,
2019).
Table 2 Summary
table of studies sperm cryopreservation to evaluate the quality embrio before
or after in vitro fertilization
|
Year |
Sample size |
Cryoprotectant agent |
Main outcome |
Conclusion |
References |
|
2021 |
There
were four main groups based on the origin of oocytes and semen; the FO/FS
group (n = 19); the FO/CrS group (n = 14); the CrO/FS group (n = 85); and the
CrO/CrS group (n = 34). |
Cryopreserved semen: glycerol 12%, egg yolk 20%
Vitrification and warming oosit by Kitazato® (Tokyo, Japan |
The CrO/CrS group had significantly lower normal day 3 cleavage rates
(55.5%), blastocyst development (24%), and implantation rates (14.5%) than
the FO/FS group. |
In an egg-sharing donation programme, frozen sperm was inserted into
vitrified oocytes, embryo developmental competence and implantation potential
were diminished. |
(Setti et al., 2021) |
|
2019 |
Chicken sperms 25week old |
Sucrose and raffinose (1 mmol, 5 mmol, 10 mmol) |
1 mmol sucrose concentration is efficient in increasing sperm plasma
membrane integrity/viability, acrosome integrity, and mitochondrial potential |
Sucrose is more efficient than raffinose in chicken sperm |
(Thananurak et al., 2019) |
|
2010 |
The sperm of C57BL/6J mice. Female and male donors were 8-to-10 weeks
old and a 12-to-15 weeks old |
R18S3 |
A combination of R18S3 with L-glutamine & MBCD in preincubation
media was able to increase fertilization (69.2%). then, the two-cell
embryonic development potential of frozen stored sperm was normal (thawed
51.5%) |
R18S3 modified with L-glutamine and MBCD is suitable for sperm
cryopreservation of frozen strain C57BL/6J mice with a high fertilization
rate. |
(Takeo & Nakagata, 2010) |
|
1997 |
Mice (B6D2F1 males; B6C3F1 females) (2 to 3 months old) |
Raffinose and glycerol were added EY at 37oC |
Development of embryos produced in vitro with spermatozoa frozen to
-196oC significantly differed between frozen and fresh. Cleavage
rate (68.8%) and into blastocysts (61.5%) with frozen sperm |
In frozen mice sperm, the combining of raffinose and glycerol can
increase the success of in vitro fertilization and the potential for
embryonic development. |
(Songsasen et al., 1997) |
Abbreviations: ICSI =
intracytoplasmic sperm injection; CrO=cryopreserved oosit; CrS=cryopreserved
semen; FO=Fresh oosit; FS=Fresh semen; R18S3=18% raffinose pentahydrate and 3%
skim milk; methyl-b-cyclodextrin MBCD EY=egg yolk
Table
2 shows, that currently various methods have been developed to improve the
quality of embryos from frozen stored spermatozoa. In this case the authors
will develop cryoprotectant modifications to suppress cell injury and improve
embryo quality.
3.3. Cryoprotectant Modification
Cryopreservation occurs through a
decrease in temperature to a level below the normal temperature at which all
biochemical reactions take place. This proved successful because all the normal
functions of the cells were preserved. The cryopreservation process exposes
cells to stress caused by osmotic imbalance as well. This creates an osmotic
pressure, causing the solvent to flow across the plasma membrane, from the
inside to the outside of the cell. The gradient results in the extracellular
need for water leading to a reduction in cell volume and further dehydration,
an essential process in protecting cells from intracellular ice formation,
which can cause cell deaths.
Cell injury in cryopreservation is often
associated with intracellular ice formation, and slow cooling causes osmotic
changes due to the effects of exposure to highly concentrated intra- and
extracellular solutions or mechanical interactions between cells and
extracellular ice. The procedures involved in the freezing/thawing of
spermatozoa cause cell damage to temperature oscillations, oxidative injury,
ice crystal formation, plasma membrane damage, DNA damage, cryoprotectant
toxicity, and osmotic stress. Freezing causes changes in sperm structure, sperm
function, and sperm lipids (Pini et al., 2018).
Therefore, to minimize the cellular damage that arises, in large part, from the
effects of these solutes, it is important to use an added or combined
cryoprotectant and antioxidant.
The
modified cryoprotectant is a substance that must be present in the
cryopreservation medium to minimize damage physical and chemical stress on
spermatozoa cells resulting from the cooling, freezing and thawing processes.
The modified cryoprotectant used is a combination of glycerol and raffinose.
Permeable cryoprotectants can pass through the plasma membrane to inhibit the
formation of ice crystals and reduce membrane/protein damage while reducing
cytotoxic injuries. DMSO and glycerol are the most common CPAs used to freeze
sperm cells but have toxic effects making them unsuitable for many clinical
applications. Therefore, new non-toxic CPAs should be developed. Glycerol is a
typical cryoprotectant that is frequently used to store sperm cells. Glycerol
helps protect cells by penetrating cell membranes due to its small size and
starting to affect water molecules (Best,
2015; Pegg, 2007).
Combining glycerol with non-permeating cryoprotectants (egg yolk,
raffinose, fructose, sucrose, or trehalose) appears to be the optimal method
for reducing the concentration of glycerol and its negative effects in rams
sperm (Rostami
et al., 2020), buffalo (Iqbal
et al., 2018) and bull (Hu
et al., 2010).
Survival
of animals under significant environmental stress often requires two primary
strategies: (a) preservation of cell macromolecules via stabilizing/protective
preservation mechanism and (b) inducing the hypometabolic state to reduce
energy expenditure and prioritize essential vital functions (Storey
& Storey, 2013). These preservation strategies are crucial
in extending the lifespan of cellular components. Two protein groups involved
and crucial in cellular stress response are chaperones and antioxidants (Kultz,
2005). Chaperones are constitutively present in cells and tightly
regulated under stressful environmental conditions while others are induced by
stress such as Heat Shock Protein (HSP). The HSP family were first defined by
their molecular mass: Hsp100, Hsp90, Hsp70, Hsp60, Hsp40, and small Hsps
(sHsps) (sizes < 30kDa) (Bai
et al., 2019; Schmitt et al., 2007; Zhang et al., 2015). Antioxidants are capable of
overcoming the ROS problem. In addition, antioxidants used in semen
cryopreservation are vitamins (Vitamin C and
E), nanocompounds (fullerenol), amino acids (L-arginine and melatonin),
minerals (selenite, selenocystine, and selenomethionine), natural (quercetin
and resveratrol) and synthetic compounds (Trolox, butylated hydroxytoluene) and
miscellaneous (pyruvate, butylated hydroxyanisole, n-propyl gallate (n-PG),
deferoxamine mesylate dimethyl sulfoxide and glycerol), and enzyme or
enzyme-based formulas (superoxide dismutase, glutathione, catalase, cytochrome
c, and glutathione peroxidase) (Moradi
et al., 2020). Higher concentration of ROS were detected
in human, ruminant and canine spermatozoa during cooling to 5°C (Santiani
et al., 2014) and following cryopreservation (Kim
et al., 2010) when compared to fresh spermatozoa. Table 3
shows various attempts to overcome cryopreservation-induced cell injury due to
cryopreservation. It is not yet known to determine the best course of action to
treat cell injury. However, efforts such as protecting spermatozoa damage with
the addition of antioxidants to minimize cell injury.
Abbreviation: ROS = reactive oxygen species, TAC = total antioxidant capacity; MMP = mitochondrial membrane potential; MDA = malondialdehyde; CPLL = carboxylated poly L-lysine; Me2SO = dimethyl sulfoxide; GFE = good freezability ejaculates; PFE = poor freezability ejaculates; PMI = plasma membrane integrity; SVPMI = supravital plasma membrane integrity; HR = hypo-resistivity; ACR-I = acrosome integrity; LPO = lipid peroxidation; iPAM = the integrity of the plasma and acrosomal membranes; TBARS = thio-barbituric acid reactive substances; 8OHdG = e 8-oxo-7,8-dihydro-20 deoxyguanosine.
Table 3 Summary
of efforts to overcome cell injury due to cryopreservation
|
Publication Year |
Sample |
Result |
Conclusion |
References |
|
2022 |
Normozoospermic samples (n = 25) |
The diluent with exosomes or microvesicles had
improved sperm motility, morphology and viability compared with untreated
samples. ROS levels decreased significantly with a decrease due to DNA
damage. TAC activity and MMP levels also increased significantly; MDA levels
and apoptotic rates remained unchanged. |
Seminal plasma microvesicles and exosomes could
protect spermatozoa from cryopreservation chilling injuries |
(Mahdavinezhad
et al., 2022) |
|
2021 |
Normozoospermic semen (n=12) |
The addition of 5% PRP significantly improves sperm
progressive motility, viability and membrane integrity after cryopreservation |
Autologous PRP has a partial protective effect on
human spermatozoa cryopreservation |
(Yan et
al., 2021) |
|
2021 |
Rabbit sperm cells |
The concentration of Me2SO (5 or 8%) in the sample
after thawing, significantly effected on the rate of total motility and
progressive motility as well as the level of live sperm and intact acrosomes. |
Supplementation of CPLL to the extender in the
presence of Me2SO improved the parameters of sperm quality and DNA integrity
after thawing |
(Küçük
et al., 2021) |
|
2020 |
Normozoospermic semen (n=21) |
After the sperm is stored frozen, there is rupture
of the head of the plasma membrane, damage to the acrosome, decompression of
the nucleus and chromatin, damaged mitochondria and disruption of the
axonema, with an irregular structure. |
Further research is needed to improve sperm
lyophilization results. In the future, using spermatozoa lyophilization can
reduce the cost of fertility preservation, because it does not need storage
space and transportation is simpler. |
(Bossi et al., 2021) |
|
2019 |
Buffalo bull (Bubalus bubalis) sperm |
Group (G5C0.75) containing 0.75% CPLL and 5%
glycerol demonstrated an increase in total motility and progressive motility
after sperm thawing, with higher plasma membrane integrity, acrosome
integrity, and MMP when compared to the control group. |
The addition of 0.75% CPLL in combination with 5%
glycerol in extender freezing improved structure, function and fertility in
vivo post-thawing (56%) |
(Tariq
et al., 2020) |
|
2019 |
Buffalo bull (Bubalus bubalis) sperm (n = 32) |
At post-thawing, D4 showed higher sperm progressive
motility, PMI, SVPMI, HR, ACR-I, and DNA-I than the control. Sperm MMP and
in-vivo fertility rate was higher in treated groups than the control |
The addition of L-tryptophan improved semen quality,
in vitro and in vivo fertility of buffalo spermatozoa after frozen storage. |
(Ahmed et al., 2020) |
|
2016 |
Ram Spermatozoa (n = 6) |
There was an increase in sperm motility in the
control group and the addition of canthaxanthin but did not affect the
production of intracellular ROS in spermatozoa, iPAM or LPO. |
The addition of 10 and 25 mM canthaxanthin which was
incubated at 37oC for 2 hours after thawing, protected ovine sperm
from kinetic changes. |
(Souza et al., 2017) |
|
2016 |
Canine sperm (n = 6) |
The GSH-20 addition group resulted in lower
acrosomal damage and better sperm quality with lower mitochondrial activity
and higher TBARS concentrations. |
The addition of 20 mM GSH increased mitochondrial
activity whereas 10 mM GSH was better in the fertility aspect by showing
acrosomal protection |
(Lucio et al. 2016) |
|
2015 |
Human sperm (n = 43) |
Cryopreservation significantly reduces viability and
motility, but with increased intracellular ROS and MDA of human sperm |
Melatonin protects sperm by counteracting
intracellular ROS and reducing the effects of MDA |
(Karimfar
et al., 2015) |
|
2015 |
Stallion sperm (n = 24) |
After frozen storage, the sperm viability of the GFE
group was significantly higher than PFE group. However, there was no
difference between GFE and PFE groups from the aspect of DNA fragmentation
and disulfide bonding in the sperm head protein. |
There was a difference in the level of reactive
oxygen species between ejaculated sperm with good and bad freeze ability
after cryopreservation but does not interfere to the sperm nucleus |
(Yeste et al., 2015) |
|
2014 |
Sea bream sperm |
The ability of AFPIII to protect sea bream sperm quality
is associated with a decrease in sperm protein profile during
cryopreservation |
The addition of AFPIII to DMSO extender improved the
protection against freezing |
(Zilli
et al., 2014) |
|
2013 |
Cat sperm (n = 18) |
PMI and sperm quality decreased significantly due to
the influence of cryopreservation |
Ejaculated cat sperm quality is better due to the
influence of 5% glycerol |
(Villaverde
et al., 2013) |
|
2009 |
Human sperm (n = 60) |
Spermatozoa DNA was not fragmented due to the
supplementation of 50 and 100 mM genistein to the cryoprotectant |
Genistein can reduce oxidative stress during cryopreservation |
(Thomson
et al., 2009) |
Although no formal conclusions
can be drawn regarding the cryopreservation of spermatozoa to obtain good
embryo quality, our results suggest that modified cryoprotectants can be an
alternative cryoprotectant compared to commercial cryoprotectants. In addition,
the use of antioxidants in spermatozoa cryopreservation can also prevent cell
damage due to the adverse effects of cryoprotectants. However, further researches still need to be
performed to investigate the cellular mechanisms.
The authors would like to extend
their appreciation to the "Center for Education Financial Services"
and "Indonesian Endowment Funds for Education" for supporting the
writer on this study.
Agarwal, A., Tvrda, E., 2017. Slow Freezing of Human Sperm. In Cryopreservation
of Mammalian Gametes and Embryos: Methods and Protocols, Methods in Molecular
Biology. New York: Springer, pp. 67–78
Ahmed, H., ahan, S., Khan, A., Khan, L., Ullah, H., Riaz, M., Ullah,
K. and Ullah, F., 2020. Supplementation of L-tryptophan (An Aromatic Amino
Acid) In Tris Citric Acid Extender Enhances Post-Thaw Progressive Motility,
Plasmalemma, Mitochondrial Membrane Potential, Acrosome, and DNA Integrities, and
In Vivo Fertility Rate of Buffalo (Bubalus Bubalis). Cryobiology, Volume
92, pp. 117–123
Bai, C., Kang, N., Zhao, J., Dai, J., Gao, H., Chen, Y., Dong, H.,
Huang, C. and Dong, Q., 2019. Cryopreservation Disrupts Lipid Rafts and Heat
Shock Proteins in Yellow Catfish Sperm. Cryobiology, Volume 87, pp.
32–39
Best, B.P., 2015. Cryoprotectant Toxicity: Facts, Issues, and
Questions. Rejuvenation Research, Volume 18(5), pp. 422–436
Bojic, S., Murray, A., Bentley, B.L., Spindler, R., Pawlik,
P., Cordeiro, J.L., Bauer, R. and De Magalhães, J.P., 2021. Winter is Coming: The
Future of Cryopreservation. BMC Biology, Volume 19(1), pp. 1–20
Bossi, R.D.L., Cabral, M., Oliveira, M., Lopes, S., Hurtado, R.,
Sampaio, M., Geber, S., 2021. Ultrastructural Analysis of Lyophilized Human
Spermatozoa. Jornal Brasileiro de Reproducao Assistida, Volume 25(3),
pp. 473–479
Critser, J.K., L.E. Mobraaten. 2000.
Cryopreservation of Murine Spermatozoa. ILAR Journal Volume 41(4), pp. 197–206
Fahy, G.M., Wowk, B., 2015. Principles of Cryopreservation by
Vitrification. In Cryopreservation and Freeze-Drying Protocols, Methods in
Molecular Biology. London, United Kingdom: Springer, pp. 21–82
Gandini, L., Pallotti, F., Paoli, D., Lenzi, A., 2017.
Cryopreservation of Spermatozoa. In Endocrinology of the Testis and Male
Reproduction. Italy: Springer International Publishing, pp. 1–1357
Guthrie, H.D., G.R. Welch. 2005. Impact of
Storage Prior to Cryopreservation on Plasma Membrane Function and Fertility of
Boar Sperm. Theriogenology, Volume 63(2 SPEC. ISS.), pp. 396–410
Hamidi, N. 2010. Studi Inhibisi Formasi Kristal Es Dengan
Krioprotektan Sukrosa dan Gliserol (Study of Ice Crystal Formation Inhibition
with Sucrose and Glycerol Cryoprotectants). Jurnal Rekayasa Mesin, Volume
1(1), pp. 21–26
Hanifah, N.F., 2020. Pengaruh Konsentrasi Gliserol dalam Pengencer
Tris Terhadap Kualitas Spermatozoa Kambing Sapera Before Freezing (Effect of
Glycerol Concentration in Tris Diluent on Spermatozoa Quality of Sapera Goat
Before Freezing (Effect of Glycerol Concentration in Tris Diluent on
Spermatozoa Quality of Sapera Goat Before Freezing). Jurnal Medik Veteriner, Volume 3(2), p.
154
Höfner, L., Luther, A.M., Waberski, D., 2020. The Role of Seminal
Plasma in The Liquid Storage of Spermatozoa. Animal Reproduction Science,
Volume 220, p. 106290
Hu, J.H., Zan, L.S., Zhao, X.L., Li, Q.W., Jiang, Z.L., Li, Y.K.,
Li, X., 2010. Effects of Trehalose Supplementation on Semen Quality and
Oxidative Stress Variables in Frozen-Thawed Bovine Semen. Journal of animal
Science, Volume 88(5), pp. 1657–1662
Iqbal, S., Naz, S., Ahmed, H., Andrabi, S.M.H., 2018. Cryoprotectant
Effect of Trehalose in Extender on Post-Thaw Quality and in Vivo Fertility of
Water Buffalo (Bubalus Bubalis) Bull Spermatozoa. Andrologia, Volume 50,
pp. 1–6
Jang, T.H., Park, S.C., Yang, J.H., Kim, J.Y., Seok, J.H., Park,
U.S., Choi, C.W., Lee, S.R., Han, J., 2017. Cryopreservation and Its Clinical
Applications. Integrative Medicine Research, Volume 6(1), pp. 12–18
Morris, G.J., Acton, E., Murray, B.J. and Fonseca, F., 2012. Freezing
Injury: The Special Case of The Sperm Cell. Cryobiology, Volume 64(2),
pp. 71–80
Karimfar, M.H., Niazvand, F., Haghani, K., Ghafourian, S., Shirazi,
R., Bakhtiyari, S., 2015. The Protective Effects of Melatonin Against
Cryopreservation-Induced Oxidative Stress in Human Sperm. International Journal of Immunopathology
and Pharmacology, Volume 28(1), pp. 69–76
Kim, S.H., Yu, D.H., Kim, Y.J., 2010. Effects of Cryopreservation on
Phosphatidylserine Translocation, Intracellular Hydrogen Peroxide, and DNA
Integrity in Canine Sperm. Theriogenology, Volume 73(3), pp. 282–292
Kitporntheranunt, M., Om-Songkram, S., Srichawana, K., 2017. A
Comparison of Glycerol Egg Yolk Citrate and Sperm Freeze Medium in Human Sperm
Cryopreservation. Journal of the Medical Association of Thailand, Volume
100(10), pp. S142–S147
Küçük, N., Raza, S., Matsumura, K., Uçan, U., Serin, ?., Ceylan, A.,
Aksoy, M., 2021. Effect of Different Carboxylated Poly L-Lysine and Dimethyl
Sulfoxide Combinations on Post Thaw Rabbit Sperm Functionality and Fertility. Cryobiology,
Volume 102, pp. 127–132
Kultz, D., 2005. Molecular and Evolutionary Basis of The Cellular
Stress Response. Annual Review of
Physiology, Volume 67, pp. 225–257
Lestari, S.W., 2019. Journal of Global Pharma Technology Sperm DNA
Fragmentation and Chromatin Maturation of Prepared Sperm with ALA
Supplementation: The Effect of Embryo Development Quality.
Li, Y.X., Zhou, L., Lv, M.Q., Ge, P., Liu, Y.C., Zhou, D.X., 2019. Vitrification
and Conventional Freezing Methods in Sperm Cryopreservation: A Systematic
Review and Meta-Analysis. European Journal of Obstetrics and Gynecology and
Reproductive Biology, Volume 233, pp. 84–92
Lucio, C.D.F., Silva, L.C.G., Regazzi, F.M., Angrimani,
D.D.S.R., Nichi, M., Assumpção, M.E.O., Vannucchi, C.I., 2016. Effect of Reduced Glutathione (GSH) in Canine
Sperm Cryopreservation: In Vitro and in Vivo Evaluation. Cryobiology, Volume
72(2), pp. 135–140
Mahdavinezhad, F., Gilani, M.A.S., Gharaei, R., Ashrafnezhad, Z.,
Valipour, J., Nashtaei, M.S., Amidi, F., 2022. Protective Roles of Seminal
Plasma Exosomes and Microvesicles During Human Sperm Cryopreservation. Reproductive
BioMedicine Online, Volume 45(2), pp. 341–353
Miyagi-Shiohira C, Kurima K, Kobayashi N, Saitoh, I., Watanabe, M.,
Noguchi, Y., Matsushita, M., Noguchi, H., 2015. Cryopreservation of
Adipose-Derived Mesenchymal Stem Cells. Cell Medicine, Volume 8(1-2),
pp. 3–7
Moradi, M., Karimi, I., Ahmadi, S., Mohammed, L.J., 2020. The
Necessity of Antioxidant Inclusion in Caprine and Ovine Semen Extenders: A
Systematic Review Complemented with Computational Insight. Reproduction in
Domestic Animals, Volume 55(9), pp. 1027–1043
Ni’mah, H., Rochmadi, R., Woo, E.M., Widiasih, D.A., Mayangsari,
S., 2019. Preparation and Characterization of Poly (L-Lactic Acid) Films
Plasticized with Glycerol and Maleic Anhydride. International Journal of
Technology, Volume 10(3), pp. 531–540
Pegg, D.E., 2007. Principles of Cryopreservation. In Cryopreservation
and Freeze-Drying Protocols. Methods in Molecular Biology. Totowa (NJ):
Humana Press, pp. 39–75
Pegg, D.E., 2015. Principles of Cryopreservation. In Cryopreservation
and freeze-drying protocols. London, United Kingdom: Springer, pp. 3–19
Pelufo, V., Armengol, M.L., Malcotti, V., Venturino, A. and
Aisen, E.G.,2015. Effects of Glycerol and Sugar Mixing Temperature on The
Morphologic and Functional Integrity of Cryopreserved Ram Sperm. Theriogenology,
Volume 83(1), pp. 144–151
Peris-Frau, P., Soler, A.J., Iniesta-Cuerda, M., Martín-Maestro,
A., Sánchez-Ajofrín, I., Medina-Chávez, D.A., Fernández-Santos, M.R.,
García-Álvarez, O., Maroto-Morales, A., Montoro, V., Garde, J.J., 2020. Sperm Cryodamage in
Ruminants: Understanding the Molecular Changes Induced by The Cryopreservation
Process to Optimize Sperm Quality’, International Journal of Molecular
Sciences, Volume 21(8), p. 2781
Pini, T., Leahy, T., de Graaf, S.P., 2018. Sublethal sperm freezing
damage: Manifestations and solutions. Theriogenology, Volume 118, pp.
172–181
Rostami, B., Ebrahimi, D., Sadeghipanah, H., Masoumi, R., Shahir, M.,
2020. Effects of Supplementation of Tris-Egg Yolk Extender with Different
Sugars and Antioxidants on Freezability of Ram Semen. Cryobiology, Volume
92, pp. 62–66
Santiani, A., Evangelista, S., Sepúlveda, N., Risopatrón, J.,
Villegas, J.S.R., 2014. Addition of Superoxide Dismutase Mimics During Cooling
Process Prevents Oxidative Stress and Improves Semen Quality Parameters In
Frozen/Thawed Ram Spermatozoa. Theriogenology, Volume 82, pp. 884–889
Schmitt, E., Gehrmann, M., Brunet, M., Multhoff, G., Garrido,
C., 2007. Intracellular and Extracellular Functions of Heat Shock
Proteins: Repercussions in Cancer Therapy. Journal of Leukocyte Biology,
Volume 81(1), pp. 15–27
Setti, A.S., Braga, D.P.D.A.F., Iaconelli, A., Borges, E., 2021. Cryopreservation
of Both Male and Female Gametes Leads to Reduced Embryo Development and
Implantation Potential. Zygote, Volume 29(5), pp. 377–382
Sieme, H., Oldenhof, H., Wolkers, W.F., 2015. Sperm Membrane
Behaviour during Cooling and Cryopreservation. Reproduction in Domestic
Animals, Volume 50, pp. 20–26
Sieme, H., Oldenhof, H., Wolkers, W.F., 2016. Mode of Action of
Cryoprotectants for Sperm Preservation. Animal Reproduction Science, Volume
169, pp. 2–5
Singer ST, Sweeters N., Vega, O., Higa A., Vichinsky, E.C.M., 2010.
Fertility Potential in Thalassemia Major Women: Current Findings and Future
Diagnostic Tools. Annals of the New York Academy of Sciences, Volume 1202(1),
pp. 226–230
Songsasen, S.P., Leibo, N., 1997. Cryopreservation of Mouse
Spermatozoa I. Effect of Seeding on Fertilizing Ability of Cryopreserved
Spermatozoa. Cryobiology, Volume 35, pp. 240–254
Souza, H.M., Arruda, L.C., Monteiro, M.M., Nery, I.H., Araújo
Silva, R.A., Batista, A.M., Guerra, M.M.P., 2017. The Effect of
Canthaxanthin on the Quality of Frozen Ram Spermatozoa. Biopreservation and
Biobanking, Volume 15(3), pp. 220–227
Storey, Kenneth B., and Janet M. Storey.
2013. Molecular Biology of Freezing Tolerance. Comprehensive Physiology, Volume 3(3), pp. 1283–1308
Storey, K.B., Storey, J.M., 2013. Molecular biology of freezing
tolerance. Comprehensive Physiology, Volume 3(3), pp. 1283–1308
Sulistyo, H., Priadana, D.P., Fitriandini, Y.W., Ariyanto, T., Azis,
M.M., 2020. Utilization of Glycerol by Ketalization Reactions with Acetone to
Produce Solketal Using Indion 225 Na as Catalyst. International Journal of
Technology, Volume 11(1), pp. 190–199
Sutijan, Wahyudi, S., Ismail, M.F., Mustika, P.C.B., Astuti,
W., Prasetya, A., Petrus, H.T.B.M., 2022. Forward Osmosis to Concentrate
Lithium from Brine: The Effect of Operating Conditions (pH and Temperature). International
Journal of Technology, Volume 13(1), pp. 136–146
Sztein, J.M., 2001. Comparison of Permeating and Nonpermeating
Cryoprotectants for Mouse Sperm Cryopreservation. Cryobiology, Volume 42(1),
pp. 28–39
Sztein, J.M., Takeo, T., Nakagata, N., 2018. History of
Cryobiology, with Special Emphasis in Evolution of Mouse Sperm Cryopreservation.
Cryobiology, Volume 82, pp. 57–63
Takeo, T., Nakagata, N., 2010. Combination Medium of Cryoprotective
Agents Containing L-Glutamine and Methyl-?-Cyclodextrin in A Preincubation
Medium Yields A High Fertilization Rate for Cryopreserved C57BL/6J Mouse Sperm.
Laboratory Animals, Volume 44(2), pp. 132–137
Tariq, A., Ahmad, M., Iqbal, S., Riaz, M.I., Tahir, M.Z., Ghafoor,
A., Riaz, A., 2020. Effect of Carboxylated Poly L-Lysine as A Cryoprotectant on
Post-Thaw Quality and in Vivo Fertility of Nili Ravi Buffalo (Bubalus Bubalis)
Bull Semen. Theriogenology, Volume 144, pp. 8–15
Thananurak, P., Chuaychu-Noo, N., Thélie, A., Phasuk, Y.,
Vongpralub, T., Blesbois, E., 2019. Sucrose Increases The Quality and
Fertilizing Ability of Cryopreserved Chicken Sperms in Contrast to Raffinose. Poultry
Science, Volume 98(9), pp. 4161–4171
Thomson, L.K., Fleming, S.D., Aitken, R.J., De Iuliis, G.N.,
Zieschang, J.A., Clark, A.M., 2009. Cryopreservation-Induced Human Sperm DNA
Damage is Predominantly Mediated by Oxidative Stress Rather Than Apoptosis. Human
Reproduction, Volume 24(9), pp. 2061–2070
Varisli, O., Uguz, C., Agca, C., Agca, Y., 2009. Effect of Chilling
on The Motility and Acrosomal Integrity of Rat Sperm in The Presence of Various
Extenders. Journal of the American Association for Laboratory Animal Science,
Volume 48(5), pp. 499–505
Villaverde, A.I.S.B., Fioratti, E.G., Penitenti, M., Ikoma, M.R.,
Tsunemi, M.H., Papa, F.O., Lopes, M.D., 2013. Cryoprotective Effect of
Different Glycerol Concentrations on Domestic Cat Spermatozoa. Theriogenology,
80(7), pp. 730–737
Wessel, M.T., Ball, B.A., 2004. Step-Wise Dilution for Removal of
Glycerol from Fresh and Cryopreserved Equine Spermatozoa. Animal
Reproduction Science, Volume 84(1–2), pp. 147–156
Whaley, D., Damyar, K., Witek, R.P., Mendoza, A., Alexander, M., Lakey,
J.R., 2021. Cryopreservation: An Overview of Principles and Cell-Specific
Considerations. Cell Transplantation, Volume 30, pp. 1–12
Yan, B., Zhang, Y., Tian, S., Hu, R., Wu, B., 2021, Effect of
Autologous Platelet-Rich Plasma on Human Sperm Quality during Cryopreservation.
Cryobiology, Volume 98, pp. 12–16
Yang, H., Ramstein, J., Smith, J., 2019. Non-Oncologic Indications
for Male Fertility Preservation. Current Urol Reports, 20(9), pp. 1–6
Yeste, M., Estrada, E., Rocha, L.G., Marín, H., Rodríguez?Gil, J.E.,
Miró, J., 2015. Cryotolerance of Stallion Spermatozoa is Related to ROS
Production and Mitochondrial Membrane Potential Rather Than to The Integrity of
Sperm Nucleus. Andrology, Volume 3(2), pp. 395–407
Zhang, X.G., Hu, S., Han, C., Zhu, Q.C., Yan, G.J., Hu, J.H., 2015.
Association of Heat Shock Protein 90 with Motility of Post-Thawed Sperm in
Bulls. Cryobiology, Volume 70(2), pp. 164–169
Zilli, L., Beirão, J., Schiavone, R., Herraez, M.P., Gnoni, A., Vilella, S., 2014. Comparative Proteome Analysis of Cryopreserved Flagella and Head Plasma Membrane Proteins from Sea Bream Spermatozoa: Effect of Antifreeze Proteins. PLoS ONE, Volume 9(6), pp. 1–10
