Isolation and Antibacterial Activity of Honey Bee Venom Bioactive from Apis cerana
Corresponding email: lischer.kenny@ui.ac.id
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6122
Lischer, K., Mustika, I.P., Sahlan, M., Guslianto, B.W., 2022. Isolation and Antibacterial Activity of Honey Bee Venom Bioactive from Apis cerana. International Journal of Technology. Volume 13(8), pp. 1778-1786
| Kenny Lischer | 1. Bioprocess Engineering, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia 2. Research Center of Biomedical Engineering |
| Indria Puti Mustika | Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia |
| Muhamad Sahlan | 1. Bioprocess Engineering, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia, 2. Research Center of Biomedical Engineerin |
| Brian Wirawan Guslianto | Bioprocess Engineering, Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI Depok, West Java 16424, Indonesia |
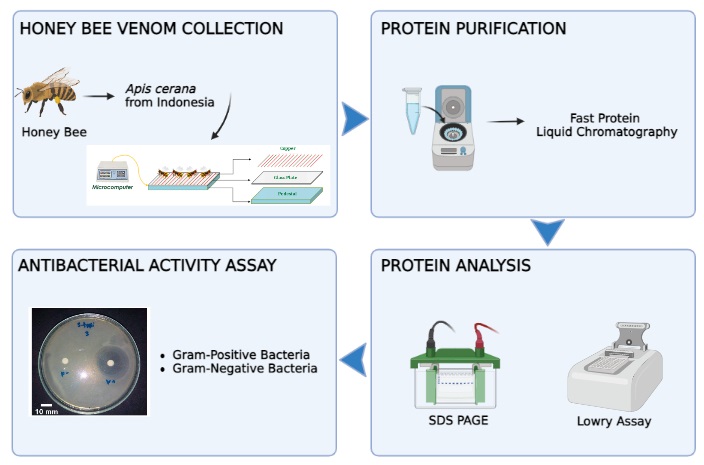
Honey bee venom from Apis species has garnered considerable attention in medicine due to its bioactive, which have antimicrobial activity. This research aims to isolate bioactive melittin and phospholipase A2 (PLA2) of honey bee venom from Indonesian Apis cerana and examine the antibacterial activity of these bioactive. Honey bee venoms were isolated from four locations using an electric shock method and purified using Fast Protein Liquid Chromatography. Purified protein was analyzed by SDS PAGE and measured by the Lowry method. Further, the antibacterial activity of melittin and PLA2 against Gram-positive (Staphylococcus aureus and Salmonella typhi) and Gram-negative (Escherichia coli) bacteria was defined by inhibition zone. Our results show that the average of all bee venoms obtained from four locations with eight sampling times was 17.1 mg proteins. Melittin was purified using gradient steps with 55%, 90%, and 100% elution buffer, while PLA2 was purified using 55% elution buffer. Melittin and PLA2 showed molecular weights of less than 7.5 kDa and 19 kDa, respectively. Protein concentrations in melittin and PLA2 were 95 µg/mL and 142 µg/mL, respectively. Among the two bioactive, only PLA2 showed antibacterial activity. The diameter of the inhibition zone was 7.76 mm when the concentration of PLA2 was 45 µg/mL against E.coli. This result showed that PLA2 from Indonesian Apis cerana has antibacterial activity and may be a promising candidate for the antibacterial agent on Gram-negative bacteria. These findings may contribute to developing new antibacterial substances derived from Indonesian Apis cerana.
Antibacterial; Apis cerana; Honey bee venom; Melittin; PLA2
Bacteria are microorganisms that are useful in a variety of areas, including food (Ahangari et al., 2021), agriculture (Afzal et al., 2019), and health (Douillard & de Vos, 2019). However, bacteria are frequently recognized as the cause of infectious diseases (Vouga & Greub, 2016). Medicines called antibiotics are used to treat bacterial infections. Antibiotic treatment may become ineffective due to antibiotic resistance (Maglangit et al., 2021). Antibiotic resistance is one of the public health issues that could threaten everyone. The impact of this issue increases the morbidity and mortality rate (Frieri et al., 2017). Failure to develop new antibiotics and inappropriate use of antibiotics are factors that accelerate the occurrence of antibiotic resistance (Nwobodo et al., 2022).
Various
studies have been conducted to discover new antibacterial compounds (Belete,
2019; León-Buitimea et al., 2020), including antibacterial
substances derived from honey bee venom (Frangieh
et al., 2019). The honey bee is an
economically beneficial insect that is extensively employed as a pollinator in
agriculture and generates various natural products (Pucca
et al., 2019). These products can be used in
medicine, such as honey (Bouacha
et al., 2018), royal jelly (Mehrnoush
& Darsareh, 2021), pollen (Sevin
et al., 2022), propolis (Sahlan
et al., 2020), and honey bee venom (Sarhan
et al., 2020). Honey bee venom is produced by
the venom glands, which are found at the back of the abdomen and are kept in
the venom reservoir (Pucca
et al., 2019).
The water
content of honey bee venom is approximately 88%. The final 12% is made up of
peptides (melittin, apamin, and adolapin), enzymes (e.g., Phospholipase A2 or
PLA2), biologically active amines, and nonpeptide components (lipid,
carbohydrates, and free amino acids). Melittin is the constituent component in
bee venom consisting of 26 amino acids. Melittin has a conformation capable of
causing damage to the double layer of lipids when binding to lipid cell
membranes (Bellik,
2015). Melittin is also known to
activate the PLA2 enzyme and work synergistically through enzyme-peptide
complexes (Soltan-Alinejad
et al., 2022). The PLA2 from bee venom Apis
mellifera is known to have antimicrobial activity in some Gram-negative
bacteria (Boutrin
et al., 2008).
Apis cerana is one of the honey bees found
in Indonesia (Hadisoesilo,
2001). Harvesting and utilization of
honey bee venom still need to be developed in Indonesia. Extraction of venom
content from honey bees must be done because of active substances that act as
antibacterial. Therefore, this study aims to isolate bioactive melittin
and PLA2 of honey bee venom from Indonesian Apis cerana and examine the
antibacterial activity of these bioactive. This study may contribute to
discovering new antibacterial substances obtained from Indonesian Apis
cerana.
2.1. Honey Bee
Venom Collection
Figure 1 Honey bee venom
collector
2.2. Protein
Purification from Honey Bee Venom
Crude bee venom was purified based on the
literature. Samples were dissolved in deionized water and centrifuged at 3000
rpm for 20 minutes. Centrifugation aims to remove impurity components that are
insoluble in water. The venom was then filtered using a 0.2 ?m microfilter to
remove small debris, pollen, and bee glue in the venom sample. Melittin and
PLA2 were then purified using a strong cation exchange column Fast Protein
Liquid Chromatography (FPLC) (Teoh
et al., 2017). The principle of protein
purification by FPLC [Amersham Pharmacia Biotech] is the difference in affinity
of the components distributed in the mobile phase and stationary phase and also
uses a small diameter stationary phase to achieve high resolution (Walls
& Walker, 2017). Melittin has an isoelectric
point (pI) of 10 (Habermehl,
2012). The selection of the column and buffer aims to bind the target protein to
the column. pH conditions below pI cause positively charged proteins and bind
to cation exchangers (Jungbauer & Hahn, 2009). In this study, 50 mM pH 6.0
sodium phosphate buffer was used as the binding buffer and 1 M NaCl in sodium
phosphate buffer was used as the elution buffer. The elution process was
carried out in stages, starting from 55%, 90%, and 100%.
2.3. Protein
Analysis
Qualitatively protein purity was tested using
the Sodium Dodecyl Sulphate Polyacrylamide Gel electrophoresis (SDS-PAGE) with
Coomassie blue staining method. Protein concentrations were tested
quantitatively using the Lowry assay (Redmile-Gordon
et al., 2013), and bovine serum albumin was
used as standard. The absorbance was read at 650 nm using a spectrophotometer
[NanoDrop].
2.4. Antibacterial Activity Assay
Staphylococcus
aureus (Gram-positive), Salmonella typhi (Gram-positive), and Escherichia
coli (Gram-negative) were used to study the antibacterial activity as these
bacteria were used in previous studies (Omar
et al., 2020). Before testing, all
microorganisms were freshly produced. The disc diffusion method assessed the
antibacterial activity of crude bee venom, melittin, and PLA2. Tetracycline,
amoxicillin, and chloramphenicol were used as positive controls for S.
aureus, S. typhi, and E. coli. All bacteria were incubated at
30o -35oC for 18-24 hours. The inhibition of microbial
growth was measured edge to edge across the zone of inhibition over the centre
of the disk.
3.1. Honey Bee Venom Collection
Honey bee venom was harvested
four times at different locations. In the first location, honey bee venom was
harvested at the original location of the bee colony, Ciburial Village.
Variation of electrical impulse shock was applied (3.2 V, 4.8 V, and 5.2 V) and
set for 5, 10, and 30 minutes. The optimum duration for honey bee venom
collection was 3.2 V for 10 minutes because not many bees fainted or died after
an electric shock. Honey bee venom was harvested at 28.2 mg.
The
second and third locations were at the Universitas Indonesia (UI). The venom
obtained was 15.6 mg and 5.8 mg. There was a significant decrease in the amount
of venom obtained in the third harvest due to the need for more time for the
colony's adaptation from Ciburial Village to the UI.
Ciburial Village represented the fourth
location, where 87.3 mg of poison was collected. The average of all acquired
poisons is 17.1 mg. A previous study obtained 24-164 mg of poison from Apis
mellifera from the harvest during the day and summer (Sanad
et al., 2013). The results obtained in this
study were less than the previous study (Table 1) because the type and size of
colonies used were different, as well as the harvest time between noon and
evening.
3.2. Protein Purification from
Honey Bee Venom
Crude bee venom was purified based on the
literature (Teoh
et al., 2017). Figure 2 shows several peaks of
absorbance values when the concentration gradients of buffer B are 0%, 55%,
90%, and 100%. The absorbance value is measured at a wavelength of 280 nm
because the protein absorbs a wavelength of 280 nm due to the presence of amino
acids, such as tryptophan, tyrosine, and cysteine (Reinmuth-Selzle
et al., 2022). Therefore, the high absorbance
value indicates the presence of proteins through the column, then measured by a
UV monitor.
Table 1 Parameters for
collection and results of honey bee venom
|
Location |
Level / Voltage |
Interval ON (s)
OFF (s) |
Time (min) |
Colony |
Sampling |
Results (mg) | |
|
(Sanad et al.,
2013) |
- |
- |
- |
- |
- |
1x |
24-164 |
|
Bandung |
10-75 / 3.2-5.2 V |
4 |
4-8 |
5-30 |
5 |
1x |
28.2 |
|
UI (EngPark) |
10 / 3.2 V |
4 |
8 |
10 |
1 |
3x |
15.6 |
|
UI (ILRC) |
10 / 3.2 V |
4 |
8 |
10 |
1 |
3x |
5.8 |
|
Bandung |
10 / 3.2 V |
4 |
8 |
10 |
6 |
1x |
87.3 |
Figure 2 FPLC
chromatogram of crude venom protein separation
Five
samples (numbers 1 to 5 in Figure 2) were taken from the FPLC fractions for
SDS-PAGE analysis (Figure 3). FPLC was used in this study because it was
designed for proteins and the availability of stationary phases in the most
common chromatography modes. Furthermore, an FPLC column costs approximately
ten times cheaper than an HPLC column (Walls
& Walker, 2017).
There are various molecular size proteins in
the lane CV because bee venom has a lot of protein content and size varies from
small to large. Two bands were observed at approximately 19 kDa in lane 1,
indicating the PLA2. It was purified using 55% elution buffer. Bellik's research showed that
40-50% of the dry weight constituents of Apis cerana honey bee venom is
melittin at 2.8 kDa and was observed in this study (lane 4 in Figure 3, less
than 7.5 kDa). It shows that melittin was purified using elusion gradient steps
ranging from 55%, 90%, and 100%. The protein bands in lanes 1 and 4 demonstrate
that PLA2 and melittin were successfully purified from honey bee venom in this
study.
The total protein concentration of the purified sample was obtained by measuring protein absorbance at 650 nm. The results of measuring protein concentrations in crude venom, melittin, and PLA2 were 1373.656; 95.432; and 142.034 ?g/ml, respectively. These results show that the largest protein concentration is crude venom because it was not purified. Protein samples were then used for antibacterial tests with various concentrations.
Figure 3 SDS PAGE gel of samples from the
protein purification. Lane MW: size of molecular weight markers in kDa; Lane M:
protein markers; Lane CV: crude venom; Lane 1 to 5: purified protein from FPLC
method
3.3. Antibacterial Activity
Assay
An assay of antibacterial
activity was performed in triplicate. The bacteria used are Staphylococcus
aureus, Salmonella typhi, and Escherichia coli. These
bacterial strains were selected to represent bioactive testing of bacteria that
are distinguished by their cell wall constituents. S. aureus and S.
typhi are Gram-positive, while E. coli is a Gram-negative. In
addition, these bacteria are widely found to be the cause of human disease
infections. Tetracycline, amoxicillin, and chloramphenicol were used as antibiotics
on positive control assays in S. aureus, S. typhi, and E. coli,
respectively.
The
bioactive tests were crude venom, melittin, and PLA2. Sample concentrations
were varied, ranging from low to high concentrations. Crude venom samples were
used at 0.1; 0.5; 1; 5; 10; and 150 ?g/mL, melittin samples were tested at 0.1;
0.5; 1; 5; 10; and 40 ?g/ml, and PLA2 sample was used only at 45 ?g/mL. The
antibacterial activity assay against Gram-positive bacteria showed that the
inhibition zones produced by tetracycline and amoxicillin were 20.1 and 33.95
mm, respectively. This study showed that there was no inhibition against
Gram-positive bacteria. The antibacterial activity test against Gram-negative
bacteria showed that the inhibition zone by chloramphenicol and PLA2 was 18.8
mm and 7.76 mm, respectively (Table 2). This study showed that antibacterial
activity is only on the bioactives of PLA2 against Gram-negative bacteria
(Figure 4). The previous study showed that PLA2 from Acanthaster plancii and
snakes showed antibacterial activity. This enzyme was known to react with
phospholipid and change it into phosphodiester and fatty acid. This reaction
yielded conformation loss on the cell membrane. Therefore, the bacteria or
cells with cell membranes become broken and inactive. Meanwhile, for other
compounds, it will use different activities. It tried to attach to the membrane
and make the hole. There is the possibility that this compound needs to
specifically bind to a specific protein absent in bacteria (Ibrahim
et al., 2013). However, further investigation
is needed. The PLA2 may be applied using various extracts from these studies (Gunawan-Puteri
et al., 2021; Haryuni et al., 2019).
The bioactive honey bee venom from Apis
mellifera has antibacterial activity against Gram-negative and
Gram-positive bacteria. Activity against Gram-negative bacteria has a more
significant inhibition zone than Gram-positive bacteria because Gram-negative
bacteria have less peptidoglycan (Zolfagharian et al., 2016). In other microorganisms, 6.29 ?g
of PLA2 from Acanthaster plancii can inhibit S.aureus
(Gram-positive) by 8.5 mm (Ibrahim
et al., 2013) and PLA2 from Pterois
volitans has antibacterial activity against S. aureus with an
inhibition zone of 14.35 mm (Sommeng
et al., 2020).
Table 2 Results of
antibacterial activity assay
|
Substance Type
|
Concentration |
Inhibition Zone Diameter (mm) |
Note | ||
|
S. aureus |
S. typhi |
E. coli | |||
|
Crude Venom |
0.1 - 150 µg/mL |
0 |
0 |
0 |
- |
|
Melittin |
0.1 - 40 µg/mL |
0 |
0 |
0 |
- |
|
PLA2 |
45 µg/mL |
0 |
0 |
7.76 |
- |
|
Amoxicillin |
1000 ppm |
not assayed |
33.95 |
not assayed |
Positive Control |
|
Tetracycline |
1000 ppm |
20.1 |
not assayed |
not assayed |
Positive Control |
|
Chloramphenicol |
30 µg |
not assayed |
not assayed |
18.8 |
Positive Control |
|
Negative Control |
- |
0 |
0 |
0 |
- |
Figure 4 Inhibition zone from antibacterial activity test on E. coli; P: PLA2 45 µg/mL; M: Melittin 40 µg/mL; C: crude venom 150 µg/mL
In
conclusion, bioactive melittin and PLA2 were isolated from
Indonesian Apis cerana. The FPLC approach, utilizing varying concentrations of elution
buffer, is an excellent way to purify melittin and PLA2 from bee venom.
Melittin was purified using elusion gradient steps ranging from 55%; 90%; and
100%, while PLA2 was purified using 55% elution buffer. Melittin and PLA2 were examined
for antibacterial activity against Gram-positive (S.
aureus and S. typhi) and Gram-negative (E. coli) bacteria.
The result suggests that antibacterial activity is only on the PLA2 against E.coli
by producing an average value of inhibition zone of 7.76 mm.
This
research was supported by grant PUTI Q2 2020 (BA-776/UN2.RST/PPM.00.03.01/2021)
from Universitas Indonesia.
Afzal,
I., Shinwari, Z.K., Sikandar, S., Shahzad, S., 2019. Plant Beneficial Endophytic Bacteria:
Mechanisms, Diversity, Host Range and Genetic Determinants. Microbiological Research, Volume 221, pp. 36–49
Ahangari, H., Yazdani, P., Ebrahimi, V.,
Soofiyani, S.R., Azargun, R., Tarhriz, V., Eyvazi, S., 2021. An Updated Review on Production of Food Derived Bioactive
Peptides; Focus on the Psychrotrophic Bacterial
Proteases. Biocatalysis and Agricultural Biotechnology, Volume 35, p. 102051
Belete, T.M., 2019. Novel Targets to Develop New Antibacterial Agents and Novel Alternatives to Antibacterial Agents. Human
Microbiome Journal, Volume 11, p. 100052
Bellik, Y., 2015. Bee Venom: Its Potential Use in
Alternative Medicine. Anti-Infective Agents, Volume 13(1), pp. 3–16
Bouacha, M., Ayed, H., Grara, N., 2018. Honey Bee as Alternative Medicine to Treat Eleven
Multidrug-Resistant Bacteria Causing Urinary Tract Infection During Pregnancy. Scientia
Pharmaceutica, Volume 86(2), p. 14
Boutrin, M.CF., Foster, H. A., Pentreath,
V.W., 2008. The
Effects of Bee (Apis
Mellifera) Venom Phospholipase A2 on Trypanosoma Brucei Brucei and Enterobacteria. Experimental Parasitology, Volume 119(2), pp. 246–251
Douillard, F.P., de Vos, W.M., 2019. Biotechnology of
Health-Promoting Bacteria. Biotechnology Advances, Volume 37(6), p. 107369
Frangieh, J., Salma, Y., Haddad, K., Mattei,
C., Legros, C., Fajloun, Z., El Obeid, D., 2019.
First Characterization of the Venom from Apis Mellifera Syriaca, A Honeybee from the Middle East Region. Toxins,
Volume 11(4), p. 191
Frieri, M., Kumar, K., Boutin, A., 2017. Antibiotic Resistance. Journal of
Infection and Public Health, Volume 10(4), pp. 369–378
Gunawan-Puteri, M.D.P.T., Kato, E.,
Rahmawati, D., Teji, S., Santoso, J.A., Pandiangan, F.I., Nion, Y.A., 2021. Post-harvest and Extraction
Conditions for the Optimum Alpha Glucosidase Inhibitory Activity of Stenochlaena
Palustris. International Journal of Technology, Volume 12(3), pp. 649–660
Habermehl, G., 2012. Venomous Animals and Their Toxins. Springer Science & Business Media
Hadisoesilo, S., 2001. Review?: The Diversity of Indigenous
Honey Bee Species of Indonesia. Biodiversitas Journal of Biological
Diversity, Volume 2(1), pp. 123–128
Haryuni, Dewi, T.S.K., Suprapti, E., Rahman,
S. F., Gozan, M., 2019. The
Effect of Beauveria Bassiana on the Effectiveness of Nicotiana Tabacum Extract
as Biopesticide Against Hypothenemus Hampei to Robusta Coffee. International
Journal of Technology, Volume 10(1), pp. 159–166
Ibrahim, F., Widhyastuti, N., Savitri, IK.
E., Sahlan, M.,Wijanarko, A., 2013. Antibacterial Investigated of Phospholipase A2 from the Spines
Venom of Crown of Thorns Starfish Acanthaster
planci. International Journal of Pharma and Bio Sciences, Volume 4(2), pp. B1–B5
Jungbauer, A., Hahn, R., 2009. Chapter 22 Ion-Exchange
Chromatography. In R. R. Burgess & M. P. B. T.-M. in E. Deutscher
(Eds.), Guide to Protein Purification, 2nd Edition, Academic Press.
León-Buitimea, A., Garza-Cárdenas, C.R.,
Garza-Cervantes, J.A., Lerma-Escalera, J.A., Morones-Ramírez, J.R., 2020. The Demand for New Antibiotics: Antimicrobial
Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in
Antibacterial Agent Design. Frontiers in Microbiology, Volume 11, pp. 1–10
Maglangit, F., Yu, Y., Deng, H., 2021. Bacterial Pathogens: Threat or Treat (a Review on Bioactive Natural Products from Bacterial Pathogens). Natural
Product Reports, Volume 38(4), pp. 782–821
Mehrnoush, V., Darsareh, F., 2021. Royal Jelly for Genitourinary Syndrome of Menopause: A Randomized
Controlled Trial. Gynecology and Obstetrics Clinical Medicine, Volume 1(4), pp. 211–215
Nwobodo, D.C., Ugwu, M. C., Oliseloke Anie, C.,
Al-Ouqaili, M. T. S., Chinedu Ikem, J., Victor Chigozie, U., Saki, M., 2022. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. Journal of Clinical
Laboratory Analysis, Volume 36(9), pp. 1–10
Omar, M.S., Sanif, M.N.M.N.M., Ali, N.H.S.O.,
Hamid, M.H.SA., Taha, H., Mahadi, A.H., Soon, Y.W., Ngaini, Z., Rosli,
M.Y.H.,Usman, A., 2020.
Synthesis of Schiff Base Encapsulated ZnS Nanoparticles: Characterization and
Antibacterial Screening. International Journal of Technology, Volume 11(7), pp. 1309–1318
Pucca, M.B., Cerni, F.A., Oliveira, I.S.,
Jenkins, T.P., Argemí, L., Sørensen, C.V., Ahmadi, S., Barbosa, J.E., Laustsen,
A.H., 2019. Bee
Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Frontiers
in Immunology, Volume 10, pp. 1–15
Redmile-Gordon, M.A., Armenise, E., White, R.P., Hirsch, P.R., Goulding, K.W.T., 2013. A Comparison of Two Colorimetric Assays, Based
Upon Lowry and Bradford
Techniques, to
Estimate Total Protein in Soil Extracts. Soil Biology and Biochemistry, Volume 67, pp. 166–173
Reinmuth-Selzle, K., Tchipilov, T., Backes,
A.T., Tscheuschner, G., Tang, K., Ziegler, K., Lucas, K., Pöschl, U.,
Fröhlich-Nowoisky, J., Weller, M.G., 2022. Determination of the Protein Content of Complex Samples by Aromatic Amino Acid Analysis, Liquid
Chromatography-UV Absorbance, and Colorimetry. Analytical and Bioanalytical Chemistry, Volume 414(15), pp. 4457–4470
Sahlan, M., Al Faris, M.NH., Aditama, R.,
Lischer, K., Khayrani, A.C. Pratami, D.K., 2020. Molecular Docking of South Sulawesi Propolis Against Fructose
1,6-Bisphosphatase as a Type 2 Diabetes Mellitus Drug. International Journal
of Technology, Volume 11(5), pp. 910–920
Sanad, Reda, E., Mohanny., Karem M., 2013. The Efficacy of a New Modified
Apparatus for Collecting Bee Venom in Relation to Some Biological Aspects of
Honeybee Colonies. Journal of American Science, Volume 9(10), pp. 1545–1003
Sarhan, M., El-Bitar, A.M.H., Hotta, H., 2020. Potent Virucidal Activity of Honeybee “Apis Mellifera”
Venom Against Hepatitis C Virus. Toxicon, Volume 188, pp. 55–64
Sevin, S., Tutun, H., Yipel, M., Aluç, Y.,
Ekici, H., 2022.
Concentration of Essential and Non-Essential Elements and Carcinogenic/Non-Carcinogenic Health Risk Assessment of Commercial Bee Pollens from
Turkey. Journal of Trace Elements in Medicine and Biology, Volume 75, p. 127104
Soltan-Alinejad, P., Alipour, H., Meharabani,
D., Azizi, K., 2022.
Therapeutic Potential of Bee and Scorpion Venom Phospholipase A2 (PLA2): A
Narrative Review. Iranian Journal of Medical Sciences, Volume 47(4), pp. 300–313
Sommeng, A.N., Ramadhan, M.Y.A., Larasati,
R., Ginting, M.J., Sahlan, M., Hermansyah, H.,
Wijanarko, A., 2020. Extraction of PLA2 and Antibacterial Activity Test of Lionfish (Pterois Volitans)
Spine Venom. In: The 3rd
International Conference on Natural Products and Bioresource Sciences – 2019
23-24 October 2019, Tangerang, Indonesia
Teoh, A.C.L., Ryu, K.H. Lee, E.G., 2017. One-step Purification of Melittin Derived from Apis Mellifera Bee
Venom. Journal of Microbiology and Biotechnology, Volume 27(1), pp. 84–91
Vouga, M., Greub, G., 2016. Emerging Bacterial Pathogens: The Past and Beyond. Clinical
Microbiology and Infection, Volume 22(1), pp. 12–21
Walls, D., Walker, J.M., 2017. Protein Chromatography. Springer Link
Zolfagharian, H., Mohajeri, M., Babaie, M., 2016. Bee
Venom (Apis Mellifera) an Effective Potential Alternative to Gentamicin
for Specific Bacteria Strains: Bee Venom
an Effective Potential for Bacteria. Journal of Pharmacopuncture, Volume 19(3), pp. 225–230
