Biotransformation of Methylene Blue by Mixed Fungal Cultures of Gloeophyllum trabeum and Aspergillus oryzae
Corresponding email: adi.spurnomo@yahoo.com
Published at : 30 Dec 2022
Volume : IJtech
Vol 13, No 8 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i8.6111
Purnomo, A.S., Prameswari, A.S., Rizqi, H.D., Alkas, T.R., Ediati, R., Kusumawati, Y., 2022. Biotransformation of Methylene Blue by Mixed Fungal Cultures of Gloeophyllum trabeum and Aspergillus oryzae. International Journal of Technology. Volume 13(8), pp. 1768-1777
| Adi Setyo Purnomo | Department of Chemistry, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember (ITS), Kampus ITS Sukolilo, Surabaya, 60111, Indonesia |
| Adelia Sabilah Prameswari | Department of Chemistry, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember (ITS), Kampus ITS Sukolilo, Surabaya, 60111, Indonesia |
| Hamdan Dwi Rizqi | Department of Chemistry, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember (ITS), Kampus ITS Sukolilo, Surabaya, 60111, Indonesia |
| Taufiq Rinda Alkas | Department of Chemistry, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember (ITS), Kampus ITS Sukolilo, Surabaya, 60111, Indonesia |
| Ratna Ediati | Department of Chemistry, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember (ITS), Kampus ITS Sukolilo, Surabaya, 60111, Indonesia |
| Yuly Kusumawati | Department of Chemistry, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember (ITS), Kampus ITS Sukolilo, Surabaya, 60111, Indonesia |
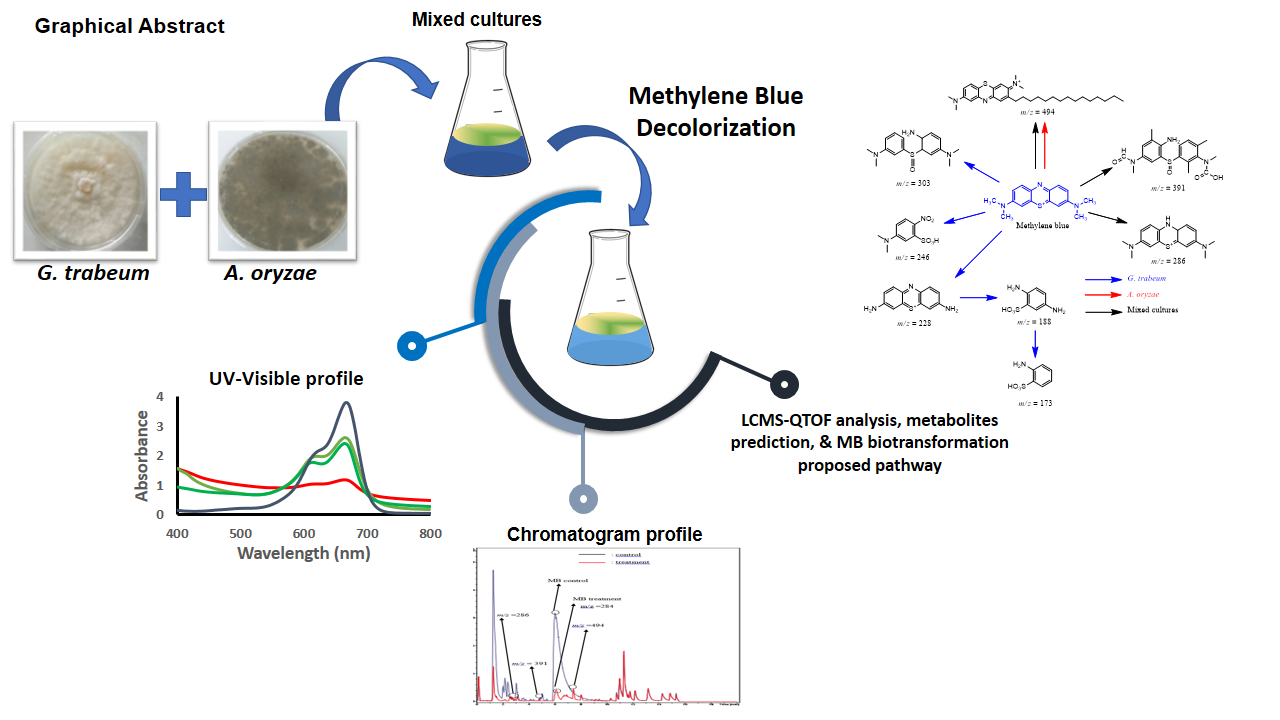
This study investigated the biotransformation of
methylene blue (MB) by mixed fungal cultures of Gloeophyllum trabeum and
Aspergillus oryzae. Equal volumes of A.
oryzae and G. trabeum cultures were applied to Erlenmeyer containing
MB and incubated at 30°C for 7 days. The change in absorbance of the MB control
solution and the solution after application, measured with a UV-Visible
spectrophotometer, was used to calculate the decolorization. The addition of A. oryzae to G. trabeum cultures showed MB biodecolorization reaching 69.34%,
greater than single cultures of G.
trabeum and A. oryzae, which were
31.50% and 36.82%, respectively. Metabolite identification from MB
biodecolorization by mixed culture using LC-QTOF-MS found the following
metabolites: C16H20N3S, C19H22N3SO4,
C31H48N3S+. The results of this
study showed that the addition of A. oryzae enhanced the percentage of
MB decolorization from G. trabeum culture.
Aspergillus oryzae; Biodecolorization; Gloeophyllum trabeum; Methylene blue; Mixed culture; Pollutants
Indonesia is one of
the main textiles producing countries in Asia, which one of
the world's top 10 exporters of textiles and textile products, along with
countries such as China, India, Thailand, Brazil, and the United States.
Indonesia's main attractions are its cheap labor force and large domestic
market (AHK Indonesien, 2022). With the increasing population
growth rate, textile production as a need for clothing increases rapidly.
This industrial
process always produces waste, especially liquid waste. Many methods have
been tried to manage textile wastewater, such as ozonation, photochemistry,
adsorption, ion exchange, floatation, and electrokinetic coagulation. Removal
efficiencies are in the range of 70-95%, but these processes still have drawbacks.
The disadvantages of these methods include the need for large areas and the
generation of a large amount of sludge, which causes problems with waste
disposal (Istirokhatun et al., 2021). However, sludge
from industrial by-products can also be processed and activated into
biosorbents (Extracellular polymeric substances/EPS) (Kistriyani et al., 2020).
Approximately 10.000 types of dyes are used in the textile industry, and during the dyeing process, 10-15% of the textile dyes used will be removed with wastewater. One of the dye often used in the textile industry is methylene blue (MB) because it is economical, easy to obtain, and has a very strong adsorption power (Pratiwi et al., 2021). Meanwhile, although MB has some advantages, it can be toxic to humans and the environment. It can also cause human health problems such as respiratory disorders, stomach disorders, blindness, and digestion and mental disorders. Furthermore, MB also triggers nausea, diarrhea, vomiting, cyanosis, shock, gastritis, jaundice, methemoglobinemia, tissue necrosis, and increased heart rate, causing premature cell death in tissues and skin/eye irritation (Khan et al., 2022). The toxicity of MB dye was studied and reported had the no observed adverse effect level (NOAEL) value of 25?mg?kg?1 for MB in rats (Bharti et al., 2019). Besides, MB had the LD50 1180 mg kg-1 (oral acute toxicity rat), LC50 18 mg L-1 (96 h, Mystus vittatus), and EC50 2.26 mg L-1 (48 h, Daphnia magna) (LabChem, 2019). The MB molecular structure is figured out in Figure 1.
Figure 1 MB molecular structure
Many methods have been applied for MB degradation including advanced
oxidation processes (AOPs), photodegradation, ozonation, oxidation with UV/H2O2,
photocatalytic degradation etc. Several studies using nanocomposites have been
carried out with excellent photodegradation results (88-100% removal), such as
CuO/Bi2O3 nanocomposites, SnO2-bentonite, TiO2/Seashell,
and ZnO-nanorods/activated carbon fibers (Khan et al., 2022). However, most of
them need high costs and require elevated energy costs.
One of the effective
methods for reducing dye wastewater is using microorganisms as a biological
activity through biodegradation. Biodegradation, also called bioremediation, is
a very broad field and the most reliable mechanism for removing organic and
inorganic pollutants from the environment is by using microorganisms (Zahari et al., 2022). One of the microorganisms used
for biodegradation is brown rot fungi (BRF). BRF produce hydroxyl radicals
generated from the Fenton reaction to degrade cellulose, hemicellulose, and
some dyes (Purnomo et al., 2022). According to Riadi et al. (2021), the initial organic compound degradation
reaction is faster and more economical with Fenton's reagent than other
chemical treatments, and the degradation yield can reach 70–99% (Riadi et al., 2021). In addition to producing
hydroxyl radicals, these fungi produce cellulase enzymes used to degrade
cellulose as a source of carbon and energy. It is an advantage of brown rot
fungi compared to white-rot fungi which only use ligninolytic enzymes as
degrading agents (Kim et al., 2014).
In a previous study
by Purnomo et al. (2021), MB biodegradation was carried
out using brown rot fungus Gloeophylum trabeum in liquid PDB
media resulting in a decolorization percentage of 71.61% for 14 days of incubation.
This result indicates that the biodegradation of MB using brown rot
fungus G. trabeum takes a long time. Hence, it is necessary to
modify the culture through mixed cultures. Aspergillus oryzae can
decolorize several types of azo dyes from aqueous solutions such as Direct Red
23 and Direct Violet 51 because A. oryzae is used as a
biosorption substrate by azo dyes (Corso et al., 2012). A. oryzae was
reported that it can be used for the remediation of hydrocarbon polluted soils (Asemoloye et al., 2020) and degrade a mycotoxin compound
(Ochratoxin A) that can contaminate agricultural products (Xiong et al., 2021). The combination of brown rot
fungus G. trabeum and filamentous fungi A. oryzae is a new
combination that has never been studied for MB degradation. This study aims to
determine the degradation ability of this combination against methylene blue
dye, predict the metabolite products, and propose the degradation pathways.
2.1. Fungi and Chemicals
The fungi
used in this study include G. trabeum and A. oryzae taken from
the Microorganism Chemistry laboratory
collection. The chemicals were used such as methylene blue (SAP Chemicals),
potato dextrose agar (PDA, Merck), potato dextrose broth (PDB, Difco),
distilled water (Brataco), alcohol (70%, Brataco), and filter paper (Whatman).
2.2. Microorganisms Culture conditions
Stock cultures of G. trabeum
and A. oryzae from the collection
were taken ± 1 cm2 of mycelium and then inoculated on PDA sterile
petri dish that had been incubated statically at 30 °C for 7 days. G. trabeum and A. oryzae mycelia (diameter 1 cm) were inoculated into 9 mL of PDB
medium separately and then pre-incubated statically for 7 days at 30 °C. The
regenerated G. trabeum and A. oryzae fungi were put into a sterile
cup blender (Waring, J-SPEC LB10BUJ) containing 25 mL of sterile distilled
water and then homogenized until evenly crushed. The homogenate (1 mL) was
inoculated into Erlenmeyer containing 9 mL of PDB liquid medium using a
micropipette and then pre-incubated statically for 7 days at 30 °C (Pratiwi
et al., 2021).
2.4. Biotransformation of MB by Mixed Cultures
A. oryzae liquid culture (10
mL) was added to the pre-incubated G.
trabeum culture (10 mL), and then added 1 mL of 2000 mg/L MB (final culture
concentration 95.24 mg/L). The mixed cultures were incubated at 30 °C for 7
days. The cultures were separated by using a centrifuge at 3000 rpm for 5
minutes after 7 days, the supernatant was analyzed by using a UV-Vis
spectrophotometer. Abiotic control was made from 20 mL PDB liquid medium added
with 1 mL MB dye to reach a final concentration of 95.24 mg/L. In contrast, the
biotic control was made from mixed cultures of G. trabeum and A. oryzae
fungi which were turned off by heating with an autoclave before adding MB. The
percentage of MB dye decolorization was calculated using Equation 1.
2.5. Analysis of Biotransformation of MB and Its Metabolite Products
3.1. Biotransformation of MB by Single and Mixed Cultures
Figure 2 The absorbance profile graph of the MB
decolorization result on the 7th day
Figure 3 The decolorization of MB by all treatments
3.2. Identification of Metabolites of MB Biotransformation by Single Culture of G. trabeum and A. oryzae
Based on the chromatogram,
there was the same peak between the control and treatment at a retention time
of 5.57 min (Figure 4). Based on the QTOF-MS analysis, the two peaks have m/z
of 284 which was the m/z of the MB. This assumption was based on the research
by Rauf and colleagues in 2010 where MB had a peak m/z of 284 (Rauf et al., 2010) and Rizqi
and Purnomo (2017) also
found this peak in MB decolorization by Daedalea
dickinsii fungus. The MB peak intensity in treatment culture was lower than the MB peak
in the abiotic control. This phenomenon showed that MB had been transformed
(degraded) and the MB concentration was reduced. In the chromatogram treatment,
new peaks appeared at the retention times of 1.63, 2.64, 3.04, 4.66, and 5.40
mins. The identification of metabolite was performed based on references of
previous studies and databases (Table 1).
Figure 4 Profile Chromatogram of MB Biotransformation by G. trabeum. Black chromatogram: abiotic control (MB + PDB), while
Red: treatment chromatogram (G. trabeum)
Table 1 Metabolites of MB decolorization by G. trabeum
The chromatogram profile of MB biotransformation by
A. oryzae showed the same peak
between control and treatment at a retention time of 6 min, with m/z of 284.122
identified as MB (Figure 5). The chromatogram treatment showed a new peak at
the 5.17 min retention time, which was identified as C31H48N3S+
(Table 2).
Figure 5 Profile Chromatogram of MB
Biotransformation by A. oryzae, Black
chromatogram: abiotic control (MB + PDB), while Red: treatment chromatogram (A. oryzae)
Table 2 Metabolites of MB decolorization by A. oryzae
3.3. Identification of Metabolites of MB Biotransformation by Mixed
Cultures
The LC chromatogram showed the same peak between control and treatment
at a retention time of 5.78 mins, which identified MB (m/z 284). Based on the chromatogram in Figure 6, the MB peak on
treatment showed a lower intensity than the MB peak in control. This indicates
a decolorization process in the treatment so that the MB concentration was
reduced. The treatment chromatogram showed that new peaks appeared at the
retention times of 2.79, 4.81, and 7.44 mins (Table 3).
The LC-QTOF MS profile showed a
decrease in the intensity of MB in treatment compared to control, which
indicated a decrease in MB concentration. The appearance of several new peaks
in the treatment indicated the metabolites of MB degradation. MB biodegradation pathway was proposed, as shown in Fig.
7. G. trabeum transformed MB via 3 initial pathways were oxidation of
the sulfide group be 3-((3-dimethylamino) phenyl)
sulfinyl-N-N-dimethylbenzen1,4-diamine; oxidation be
5-(dimethylamino)-2-nitrobenzenesulfonic acid; and demethylation to
3,7-diaminophenothiazin-5-ium, then oxidation cleavage to 2,5- diamino
benzenesulfonic acid, and to 2-aminobenzenesulfonic acid.
Besides the transformation process of MB by A. oryzae, the product metabolites were N-(8-(dimethylamino)-2-pentadecyl-3H-phenothiazin-3-ylidene)-N-methylmethanamin-
ium. From the
transformation process of MB by mixed cultures, the product metabolites were
3,7-bis (dimethylamino)-9a,10 dihydrophenothiazin-5-ium;
3-2-amino-3-methyl-5-(N-methylformamido) phenyl) sulfinyl) 2,6-dimethylphenyl)
(methyl) carbamic acid; and N-
(7-(dimethylamino)-2-pentadecyl-3H-phenothiazin-3-ylidine)-N-methylmethanamin-
ium. The estimated pathway for MB degradation
using mixed culture G. trabeum and A. oryzae is shown in Figure 7. This study indicated that the
mixed culture could be used to transform MB.
Figure 6
Profile Chromatogram of MB Biotransformation by mixed cultures, Black
chromatogram: abiotic control (MB + PDB), while Red: treatment chromatogram
(mixed cultures)
Table 3 Metabolites of
MB decolorization by mixed cultures
Figure 7 MB degradation proposed pathway
by single cultures and mixed cultures
The single culture of
G. trabeum and A. oryzae decolorized methylene blue (MB) by 31.50% and 36.82%,
respectively. In comparison, the mixed cultures of G. trabeum and A. oryzae
decolorized MB by 69.34% after incubation for 7 days. Based on the LC-QTOF MS analysis,
the MB metabolite from biodecolorization by A.
oryzae was C31H48N3S+, while
that by G. trabeum were C6H8N2SO3,
C6H7NSO3, C8H10N2SO5,
C16H20N3SO, and C12H10N3S.
On the other hand, the MB metabolites by mixed cultures were C16H20N3S,
C19H22N3SO4, and C31H48N3S+.
This study indicated that mixed cultures of BRF of G. trabeum and filamentous fungus A. oryzae were effective in decolorizing MB dye.
This study was funded by the
Directorate of Research, Technology, and Community Service, Ministry of
Education, Culture, Research and Technology of Indonesia in accordance with the
World Class Research Scheme Number: 008/E5/PG.02.00.PT/2022.
AHK Indonesien, 2022.
The Indonesian Textile Industry Must Adapt Quickly to the Global Market as it
Ratifies RCEP. AHK Indonesien. Available online at
https://indonesien.ahk.de/en/infocenter/news/news-details/the-indonesian-textile-industry-must-adapt-quickly-to-the-global-market-as-it-ratifies-rcep,
Accessed on December 2, 2022
Arimoto, M.,
Yamagishi, K., Wang, J., Tanaka, K., Miyoshi, T., Kamei, I., Kondo, R., Mori,
T., Kawagishi, H., Hirai, H., 2015. Molecular Breeding of Lignin-Degrading
Brown-Rot Fungus Gloeophyllum Trabeum by Homologous Expression of
Laccase Gene. AMB Express, Volume 5(1), pp. 1–7
Asemoloye, M.D.,
Tosi, S., Daccò, C., Wang, X., Xu, S., Marchisio, M.A., Gao, W., Jonathan,
S.G., Pecoraro, L., 2020. Hydrocarbon Degradation and Enzyme Activities of Aspergillus
oryzae and Mucor irregularis Isolated from Nigerian Crude Oil-Polluted
Sites. Microorganisms, Volume 8, p. 1912
Bharti, V., Vikrant,
K., Goswami, M., Tiwari, H., Sonwani, R.K., Lee, J., Tsang, D.C.W., Kim, K.-H.,
Saeed, M., Kumar, S., Rai, B.N., Giri, B.S., Singh, R.S., 2019. Biodegradation
of Methylene Blue Dye in a Batch and Continuous Mode Using Biochar as Packing
Media. Environmental Research, Volume 171, pp. 356–364
Corso, CR., Almeida,
EJ., Santos, GC., Morao, LG., Fabris, GS., Mitter, EK., 2012. Buioremediation
of Direct Dyes in Simulated Textile Effluents by a Paramorphogenic Form of Aspergillus
oryzae. Water Science and Technology, Volume 65(8), 1490–1495
Istirokhatun, T.,
Susanto, H., Budihardjo, M.A., Septiyani, E., Wibowo, A.R., Karamah, E.F., 2021.
Treatment of Batik Industry Wastewater Plant Effluent using Nanofiltration.
International Journal of Technology, Volume 12(4), pp. 770–780
Khan, I., Saeed, K.,
Zekker, I., Zhang, B., Hendi, A.H., Ahmad, A., Ahmad, S., Zada, N., Ahmad, H.,
Shah, L.A., Shah, T., Khan, I., 2022. Review on Methylene Blue: Its Properties,
Uses, Toxicity and Photodegradation. Water, Volume 14(2), p. 242
Kim, H.M., Lee, K.H.,
Kim, K.H., Lee, D.-S., Nguyen, Q.A., Bae, H.-J., 2014. Efficient Function and
Characterization of GH10 Xylanase (Xyl10g) from Gloeophyllum trabeum in
Lignocellulose Degradation. Journal Biotechnology. Volume 172, pp. 38–45
Kistriyani, L.,
Salimin, Z., Chafidz, A., 2020. Utilization of Extracellular Polymeric
Substances (EPS) Immobilized in Epoxy Polymer as Double Ion Exchanger
Biosorbent for Removal of Chromium from Aqueous Solution. Communications in
Science and Technology, Volume 5, pp. 40–44
LabChem, 2019. Methylene
Blue Safety Data Sheet. SDS US LabChem. USA
Nabilah, B., Purnomo,
A.S., Rizqi, H.D., Putro, H.S., Nawfa, R., 2022. The Effect of Ralstonia
Pickettii Bacterium Addition on Methylene Blue Dye Biodecolorization by
Brown-Rot Fungus Daedalea Dickinsii. Heliyon, Volume 8(2), p.
e08963
Pratiwi, N.I.,
Purnomo, A.S., Rizqi, H.D., Alkas, T.R., Nawfa, R., 2021. Biodecolorization and
Biotransformation of Methylene Blue by Mixed Cultures of Brown-Rot Fungus
Gloeophyllum Trabeum and Filamentous Fungus Trichoderma Viride. In: 4th
International Seminar on Chemistry, Surabaya, Indonesia, p. 020076
Purnomo, A.S.,
Andyani, N.E.A., Nawfa, R., Putra, S.R., 2020. Fenton Reaction Involvement on
Methyl Orange Biodegradation by Brown-Rot Fungus Gloeophyllum Trabeum.
In: AIP Conference Proceedings, Volume 2237, p. 020002
Purnomo, A.S., Rizqi,
H.D., Ulfi, A., Nawfa, R., Putro, H.S., 2022. Decolorization and Transformation
of Synthetic Dye Methylene Blue by Brown-Rot Fungus Fomitopsis pinicola. Indonesian
Journal of Chemistry. Volume 22(2), 557–564
Purnomo, A.S.,
Ubaidillah, N.A., Rizqi, H.D., Nawfa, R., Putro, H.S., 2021. Decolourisation
and Degradation of Methylene Blue Dye by Brown-rot Fungus Gloeophyllum
trabeum. ASM Science Journal, Volume 16, pp. 100–106
Rauf, M.A., Meetani,
M.A., Khaleel, A., Ahmed, A., 2010. Photocatalytic Degradation of Methylene
Blue Using a Mixed Catalyst and Product Analysis by LC/MS. Chemical Engineering Journal, Volume 157, pp. 373–378
Riadi, L.,
Tanuwijaya, A.D., Je, R.R., Altway, A., 2021. Fenton’s Oxidation of Personal
Care Product (PCP) Wastewater: A Kinetic Study and the Effects of System
Parameters. International Journal of Technology, Volume 12(2), pp.
298–308
Rizqi, H.D., Purnomo,
A.S., 2017. The Ability of Brown-Rot Fungus Daedalea Dickinsii to Decolorize
and Transform Methylene Blue Dye. World Journal of Microbiology and
Biotechnology, Volume 33, p. 92
Xiong, K., Zhi, H.W.,
Liu, J.Y., Wang, X.Y., Zhao, Z.Y., Pei, P., Deng, L., Xiong, S.Y., 2021.
Detoxification of Ochratoxin a by a Novel Aspergillus Oryzae Strain and
Optimization of its Biodegradation. Revista Argentina de Microbiología,
Volume 53(1), pp. 48–58
Zahari, N.Z., Yan,
G.P., Rahim, S.A., 2022. Effects of Single and Consortia Inoculants on the
Biodegradation Efficiency of Crude Oil in Seawater. International Journal of
Technology, Volume 13(2), pp. 422–431
