Microstructure and Mechanical Properties of Magnesium ZRE1 (Mg-Zn-Zr) Alloy with Rare Earth Element (Samarium) Addition
Corresponding email: nurkamilah@uthm.edu.my
Published at : 25 Jan 2024
Volume : IJtech
Vol 15, No 1 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i1.6057
Tukiat, I.S.T., Yusuf, N.K., Khaireez, H., Al-Alimi, S., Lajis, M.A., Shamsudin, S., Ruhaizat, N.E., 2024. Microstructure and Mechanical Properties of Magnesium ZRE1 (Mg-Zn-Zr) Alloy with Rare Earth Element (Samarium) Addition. International Journal of Technology. Volume 15(1), pp. 49-62
| Ikhwan Shah Tisadi Tukiat | Sustainable Manufacturing and Recycling Technology (SMART), Advanced Manufacturing & Materials Center (AMMC), Faculty of Mechanical and Manufacturing Engineering, Universiti Tun Hussein Onn Malaysia, |
| Nur Kamilah Yusuf | Sustainable Manufacturing and Recycling Technology (SMART), Advanced Manufacturing & Materials Center (AMMC), Faculty of Mechanical and Manufacturing Engineering, Universiti Tun Hussein Onn Malaysia, |
| Haikal Khaireez | Sustainable Manufacturing and Recycling Technology (SMART), Advanced Manufacturing & Materials Center (AMMC), Faculty of Mechanical and Manufacturing Engineering, Universiti Tun Hussein Onn Malaysia, |
| Sami Al-Alimi | Sustainable Manufacturing and Recycling Technology (SMART), Advanced Manufacturing & Materials Center (AMMC), Faculty of Mechanical and Manufacturing Engineering, Universiti Tun Hussein Onn Malaysia, |
| Mohd Amri Lajis | Sustainable Manufacturing and Recycling Technology (SMART), Advanced Manufacturing & Materials Center (AMMC), Faculty of Mechanical and Manufacturing Engineering, Universiti Tun Hussein Onn Malaysia, |
| Shazarel Shamsudin | Sustainable Manufacturing and Recycling Technology (SMART), Advanced Manufacturing & Materials Center (AMMC), Faculty of Mechanical and Manufacturing Engineering, Universiti Tun Hussein Onn Malaysia, |
| Nasha Emieza Ruhaizat | Sustainable Manufacturing and Recycling Technology (SMART), Advanced Manufacturing & Materials Center (AMMC), Faculty of Mechanical and Manufacturing Engineering, Universiti Tun Hussein Onn Malaysia, |
The
influences of rare earth (RE) samarium (Sm) added contents to ZRE1 (Mg-Zn-Zr)
magnesium (Mg) cast alloy over strength properties to be investigated. Sm at
0.5, 1.0, 1.5, and 2.0 wt.% were added separately as an alloying element to
ZRE1 alloy. Optical Microscope (OM), X-ray powder diffraction (XRD), and
scanning electron microscope Energy-dispersive X-ray (SEM/EDS) were used to
investigate the microstructure of alloy, while mechanical properties
investigated include Ultimate Tensile Strength (UTS)and Micro-Hardness (MH)
tests. The result revealed that as the Sm level reached 1.5 wt.%, the grain
size decreased by 20.9 %. Additionally, UTS and Yield Strength (YS) showed
improvements of 8.5 % and 7.9 %, respectively, with the addition of 1.5 wt.% of
Sm. In addition, elongation and hardness have been improved by 32.3 % and 10.9 %
respectively at 1.5 wt.% Sm addition. Mg-Zn-Ce-Sm was formed as a new phase
upon the addition of Sm and was detected via XRD analysis. The addition of Sm
to the ZRE1 alloy had significant effects on the refinement of the material's
microstructure, leading to an increase in its mechanical and physical
properties. Therefore, the new ZRE1-RE magnesium alloy was developed.
Magnesium ZRE1 alloy; Microstructure and mechanical properties; Metal forming and materials refinement
Magnesium (Mg) alloys have great potential as engineering materials
because of their high specific strength, low density, high stiffness, excellent
electromagnetic shielding characteristics, superior damping capacity, and good
machinability (Kristanto, Gusniani, and Ratna, 2015;
Rzychon, Kielbus, and Szala, 2008). Mg alloys have sparked
enormous interest in aerospace, defense, electronics, and automotive industries
in recent years. However, Mg application in engineering is still limited due to
high material cost, poor corrosion resistance as well as low strength,
durability, fatigue life, ductility, toughness, and creep resistance (Purnama et al., 2020; Kiani et al., 2017;
Adrian, 2012). The addition of RE metal to Mg alloy could overcome its weakness
and further improve its properties.
According to (Jahedi, McWilliams, and Knezevic, 2018), the rare-earth elements containing Mg alloys could (i) increase ductility and strength while reducing the anisotropy and tension/compression asymmetry, (ii) higher resistance to creep and corrosion, (iii) improve biodegradability, (iv) reduce flammability, (v) retain high-temperature strength, (vi) improve elongation to fracture, (vii) grain boundary strengthening and (viii) improve fatigue resistance and fracture toughness. Zinc (Zn) is the second most effective and common alloying material to produce Mg alloy after aluminum (Al). The addition of zinc in Mg alloy increases room-temperature strength, increases alloy fluidity in casting, and improves corrosion resistance (Moosbrugger, 2017). In addition, recent research shows that adding RE elements to Mg-Zn alloy can further improve various mechanical and chemical properties. The most common RE metal additions in Mg-Zn alloy are Cerium, Lanthanum, Gadolinium, Neodymium, and Yttrium (Al-Alimi et al., 2021; Materialstechnology, 2019).
ZRE1 is one of
many Mg alloys available in the market nowadays. ZRE1 is an Mg alloy containing
RE metal with the addition of zinc and zirconium, forming Mg-RE-Zn-Zr (Ahmad et al., 2017a). The detailed
chemical composition of the ZRE1 alloy is shown in Table 1. ZRE1 exhibits
superior high-temperature creep resistance (Ferro,
Saccone, and Delfino, 2013) and resistance to stress relaxation compared
to that of benchmark alloy AE42 (Rzychon, Kielbus, and Szala, 2008). The presence of RE in ZRE1 makes the alloy free
of microporosity and holds up to hot cracking, which at the same time offers
good weldability (Ferro, Saccone, and Delfino, 2013).
Currently, ZRE1 is used in the aerospace industry for intermediate casings on
Tay engines, gearboxes of RB211 engines, and gearboxes of Tay engines (Materialstechnology, 2019).
Table 1 Chemical composition of the ZRE1 alloy in wt.% (Rzychon, Kielbus, and Szala, 2008)
|
Zn |
RE |
Zr |
Ni |
Si |
Cu |
Mn |
Fe |
Mg |
|
2.7 |
3.18 |
0.53 |
<0.001 |
<0.01 |
<0.01 |
0.02 |
0.002 |
balance |
Research by (Ahmad et al., 2017a)
explored the effect of Praseodymium (Pr) addition on the microstructure and
hardness of cast ZRE1 Mg alloy. They reported that 1 wt.% Pr addition on ZRE1
cast alloy reduces the grain size of ZRE1 alloy by around 37%. Meanwhile, the
addition of Pr improves the hardness value of ZRE1 by 24 %. This is due to the
effect of grain refinement and the effect of the second phase (Mg-Zn)12 RE and
intermetallic compound (Mg-Zn-Ce-Pr). In their study, Ahmad
et al. (2016) extended their research to examine the effects of
Gadolinium on the microstructure and hardness of ZRE1 cast alloy. They found
out that the addition of 3 wt.% heavy RE Gadolinium (Gd) reduce grain size by
28% and increased the volume fraction of eutectic secondary phase while at the
same time improving the hardness by 34%.
Samarium (Sm) is one of the RE elements with
huge potential to form Mg alloy. Researchers have been applied Sm as an
alloying element for various Mg alloys for a long time (Guan
et al., 2018). The addition of Sm to Mg alloy could reduce grain
size, improve corrosion resistance, enhance yield strength and ultimate tensile
strength, improve toughness, and at the same time, better elongation to Mg
alloys, as shown in Table 2. However, to the best of our knowledge, researchers
still have not given any attention to the influence of Sm addition on
microstructures and mechanical properties of as-cast ZRE1 alloy. Sm is an
orthorhombic structure with good solid-solution and precipitation strengthening
in Mg alloys (Li, Xu, and Tong, 2019).
Additionally, Sm costs are lower compared to Nd, Y, and Gd in the market (Lucas et al., 2014). Based on the Rare
Earth Metal price, Sm is the cheapest RE of all, only beaten by Ce and La in
price (Institute of Rare Earths and Strategic
Metals eV, 2020). Additionally, Sm is the seventh most abundant RE in
earth’s crust, with 7.1 ppm. Among all earth’s elements, Sm is the 40th most
abundant and widely available than silver, tin, and gold (Enghag, 2004).
Hence, researchers have conducted studies on the
effect of Sm addition to various magnesium systems such as Mg-6.0Zn-0.5Zr alloy
(ZK60) (Guan et al., 2018),
Mg-11Gd-2Y-0.6Al (Chen et al., 2019),
Mg-Al-Zn (AZ61) (Anawati, Asoh, and Ono, 2018; Chen
et al., 2015) Al-Zr-Ce (Kirman, Zulfia, and
Suharno, 2016) and Mg-5Y-2Nd-0.5Zr (Yunwei et
al., 2018). It is found the application of 1.5 wt.% Sm additive in
Mg- 6.0Zn-0.5Zr alloy (ZK60) refined the grains of the as-cast sample, while
the extruded sample shows even finer (Guan et
al., 2018). At the same time, Sm addition further improves the
strength of ZK60 alloy due to fine grain strengthening and precipitation
strengthening. This outcome is consistent with Chen
et al. (2019) findings, where they demonstrated a refined grain
structure and improved tensile strength in the microstructure and mechanical
properties of the Mg-Gd-Y-Sm-Al alloy. This corresponds to a study on the
effects of samarium addition to the AZ61 (Mg-Al-Zn) magnesium alloy, resulting
not only in a refined microstructure but also in improved ultimate tensile
strength and elongation. However, excessive Sm addition could cause the
coarsening of grain, leading to the decline of strength and plasticity (Rady et al., 2019; Al-Alimi, Lajis, and
Shamsudin, 2017).
Table 2 Previous study on RE addition to Mg alloy
|
Researcher |
Mg alloy |
RE addition |
Result |
|
Liu et al. (2016) |
Mg-Zn-Zr |
Ce, Y |
Improve YS,
UTS |
|
Rogal et al. (2019) |
E21 Mg-Gd-Nd-Zn-Zr |
Nd-Y |
Improve YS, plasticity, hardness |
|
Ahmad et al. (2017a) |
ZRE1
Mg-RE-Zn-Zr |
Pr |
Reduces grain
size, improves hardness |
|
Ahmad et al. (2016) |
ZRE1 Mg-RE-Zn-Zr |
Gd |
Increased volume fraction, improved
hardness |
|
Guan et al. (2018) |
ZK60 Mg-Zn-Zr |
Sm |
Improves
strength |
|
Chen et al. (2019) |
Mg-Gd-Y-Sm-Al |
Sm |
Improved TS, microstructure |
|
Chen et al. (2015) |
AZ61
Mg-Al-Zn-Mn |
Sm |
Refined
microstructure, improved UTS, EL |
|
Yunwei et al. (2018) |
Mg-Y-Nd-Zr |
Sm |
Improve UTS, YS and hardness |
|
Liu et al. (2021) |
AZ41
Mg-Al-Zn-Mn |
Sm |
Improve UTS,
YS and EL |
|
Rokhlin et al. (2021) |
Mg-Gd |
Sm |
Increase strength |
Samarium also has unique properties that make it useful for various
applications other than as an alloying element in metals. Chauhan, Lohra, and Langyan (2020) found that
ternary samarium (III) complexes exhibit fascinating optical properties, making
them potentially useful in bio-assays, electroluminescent devices, and liquid
lasers. Luo et al. (2019) synthesized
a novel samarium complex that emits red light and has potential as a red light
emitting material for LEDs. Meanwhile Hashmi et
al. (2019) studied the effects of samarium incorporation in ZnO thin
films and found that it affected the optical and electrical characteristics of
the films, making them suitable for use in optical devices for UV and blue
emission. Marzouk and Hammad (2020) found that samarium oxide affects the structural and optical properties
of bismuth glass, making it suitable for photonic applications. Sadeq and Morshidy (2020) showed that samarium
oxide influences the structural, optical, and electrical properties of
alumino-borate glasses, making them good optical filters. Overall, the papers
suggest that samarium has unique properties that make it useful for various
applications in optics, electronics, and materials science.
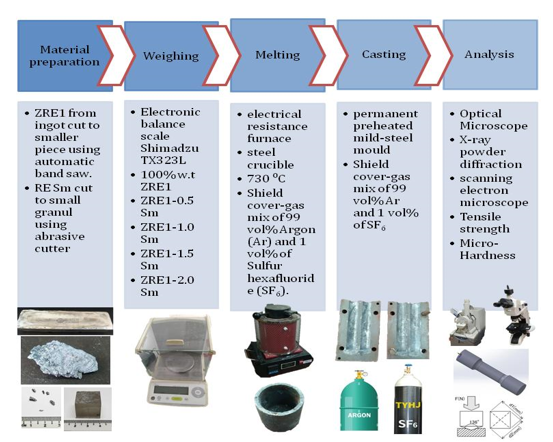
An Mg base alloy (ZRE1 Mg cast) was supplied
by ILM Ventures Ltd., Kuala Lumpur, Malaysia in ingot cast. The base alloy was
melted in an electrical resistance furnace with a steel crucible under a
cover-gas mixture of 99 vol% Argon (Ar) and 1 vol% of Sulfur hexafluoride
(SF6), both from Linde and supplied by Linde Malaysia Sdn Bhd, Kuala Lumpur,
Malaysia. Sm from Merck, Kuala Lumpur, Malaysia was added in contents of 0.5 wt.%,
1.0 wt.%, 1.5 wt.% and 2.0 wt.% separately as small pieces after the base alloy
melted at approximately 730°C. After the addition, the melt was stirred for
approximately 30 minutes to ensure stabilization, homogeneous composition, and
dissolution of the alloying elements. The molten metal was then poured into a
permanent steel mold preheated to 400°C. The cast was then left to cool down to
room temperature before being removed from the mold. The graphical abstract of
the research methodology and the melt-stir casting setup are shown in Figure 1
and Figure 2, respectively.
Figure 1
All microstructure analysis samples were cut from the center of the cast alloy. The sample was then prepared using the ASTM Standard Guide for Preparation of Metallographic Specimens (ASTM, 2011; Geels et al., 2007) grinding/polishing procedures before final etching with 4 vol% picric acid in ethanol solution. An optical microscope (Nikon Eclipse LV150NL) and image analysis software (IMT i-Solution DT v12.0) was used to measure the grain size and volume fraction. In addition, the specimens were examined using a scanning electron microscope (SEM) equipped with an energy-dispersive X-ray spectrometer (EDS) (Hitachi SU1510) and X-ray powder diffraction (XRD) (Bruker D8 Advance) for microstructure study and phase formed in the Mg alloy. A permanent preheated mild-steel mold was used to produce cylindrical tensile test specimens. The melt was neatly poured at 730 ± 5 °C into the preheated permanent steel mold after being skimmed and stirred. The castings were machined using CNC turning to achieve precise specimen dimensions based on the requirement of ASTM B557M (ASTM International, 2015).
Figure 2 Melt-stir casting setup with Argon and SF6 gas shield
XRD analysis was performed on Figure 3 and
the selected specimens to identify the phases present in the ZRE1-xSm alloys;
the results are shown in 1. It can be seen that the base alloy consisted of phase and Mg-Zn-Ce secondary intermetallic phase. The
phase was found
in a hexagonal structure, while the secondary phase was a tetragonal structure.
The position of peaks originating from Mg are exactly equivalent to Mg standard
peaks as reported by (Sheggaf et al., 2017;
Wang et al., 2014).
phase was found to possess a hexagonal
structure with crystallographic parameters of (a = 3.20936; b = 3.20936;
c = 5.21120)
while the Mg-Zn-Ce phase was tetragonal with (a = 3.83100;
b = 3.83100; c = 3.83100)
crystallographic parameters. Similar
phases were obtained by other Mg-Zn-Zr with RE addition alloys (Sheggaf et al., 2017; Liu et al., 2016).
Figure 3 XRD pattern of ZRE1-xSM
alloys
Furthermore, the XRD pattern also detected other phases in
the base ZRE1 alloy, which is MgZn2 and Zn2Zr3.
These phases form in small amounts and dissolve inside the Mg matrix of the
base alloy (Sheggaf et al., 2017; Liu et al., 2016). MgZn2 has a
hexagonal crystal structure with crystallographic parameters (a = 5.22250;
b = 5.22250; c = 8.56840) while Zn2Zr3
has a tetragonal crystal structure and (a = 7.63300; b = 7.63300;
c = 6.96500)
crystallographic parameters. The addition of the rare
earth element Sm to the ZRE1 base alloy resulted in the detection of the
MgZn(Ce,Sm) phase within the sensitivity limits of XRD, manifesting at the same
peaks as the secondary phase of the ZRE1 base alloy. The emergence of this
MgZn(Ce,Sm) phase indicates the transformation of a new phase from the
secondary phase of the ZRE1 base alloy (Sheggaf et
al., 2017). The new MgZn (Ce, Sm) intermetallic phase has a hexagonal
crystal structure with (a = 14.61900; b = 14.61900; c = 8.70800)
crystallographic parameters. Additionally, the Mg-Zn-Sm intermetallic phase
was detected at ZRE1-2.0 Sm alloy at the same peak of MgZn(Ce, Sm). This phase
shows that both phases were crystallized and combined at the alloy grain
boundaries (Sheggaf et al., 2017; Liu et al., 2016). Previous studies also
found the same result, where the MgZnRE phase was formed as a secondary
intermetallic phase (Sheggaf et al., 2017).
The addition of Sm does not change the crystalline size and
shape of before and after adding Sm. Both samples have a hexagonal
structure with crystallographic parameters of (a = 3.20936; b = 3.20936;
c = 5.21120)
and structure angle alpha 90o - beta 90o
- gamma 120o. However, there are slight changes in the crystalline
size of the MgZn2 crystal structure. Even though both samples
possess MgZn2 in a hexagonal structure, the size decreases from (a
= 5.22250; b = 5.22250; c = 8.56840)
for base ZRE1 alloy to (a
= 5.22100; b = 5.22100; c = 8.56700)
. Small changes in the c value
may be due to the addition of Sm, which has a large-sized atom in comparison to
a small atom of Mg, pushing the MgZn2 crystal compound to squeeze
slightly and squish its hexagonal structure size. According to one theory by Ke-Jie et al. (2009), when a solid
solidifies, Sm atoms are squeezed through the solid-liquid barrier, causing
constitutional supercooling and assisting in the development of a nucleus.
Furthermore, the mechanical properties of the material are
mainly caused by the type, distribution, amount, and morphology of the
intermetallic phase (Sheggaf et al., 2017).
The MgZnRE intermetallic phase has a long periodic piling ordered structure and
is coherent with the phase matrix. In Figure 4, the property-making MgZnRE
phase becomes a strengthening mechanism of MgZn alloys, thus improving
mechanical properties (Liu et al., 2016).
The strengthening of Mg alloys is due to the solid solution of the RE atoms in
borderline sizes together with secondary phase hardening of the MgZnRE
intermetallic compound (Yang et al., 2008).
The RE addition to Mg-Zn alloys formed intermetallic phases that improved the
strength of the Mg alloy. This improvement was caused by grain boundary
strengthening of the MgZnRE phase and MgZn (Ce, Sm) phase crystallized along the
grain boundaries. At the same time, the matrix phase and intermetallic phase
form a rigid atomic bonding by obstructing slip dislocation of MG-Zn-RE alloy
planes, thus strengthening the material further (Tekumalla
et al., 2014). Furthermore, both secondary phases, MgZn2
and Zn2Zr3 from ZRE1 base alloy, disappear completely
with the addition of Sm. This may be due to the addition of RE, which reduces
the solubility of Zn in the Mg matrix. As a result, the phases may not develop
or may form in small quantities, potentially beyond the detection limit of the
XRD spectrum (Huang et al., 2009).
Figure
4 shows optical microscopes taken from the ZRE1 base alloy and alloy with Sm
addition. The alloy microstructure consists of matrix (marked A) and
secondary phase crystalline along the grain boundary (marked B). The secondary
intermetallic phase forms in location B are known from XRD data as Mg-Zn-Ce,
MgZn2, and Zn2Zr3. The secondary phase at the
microstructure grain boundary shows massive morphology in a dark contrast
color. The Sm addition to the alloy changes the microstructure of the ZRE1 base
alloy. Similarly, research by (Sheggaf et al.,
2017) and (Kusrini et al., 2021)
showed that ZRE1 base alloys microstructure changes with the addition of Pr.
Figure 4 Microstructure image (a) ZRE1 base alloy and
(b-e) ZRE1 with Sm addition using optical microscope
The addition of
Sm shows a significant effect on ZRE1 grain size in Figure 5, where the
addition of 0.5 wt.% Sm reduces the grain size significantly by about 13% due
to an increase in the coherency time of the alloy (Ahmad
et
al., 2017b). Previous study shows that the dendrite
impingement rate occurs at a low pace while occurs at a higher speed for small
grain sizes (StJohn et al., 2005). The increase
of Sm content shows a reduction in the grain size of the alloy, which obtains
33.33 µm grain size of 0.5 wt.%, 31.53 µm for 1.0 wt.%, 30.43 µm for 1.5 wt.%
and 32.41 µm for 2.0 wt.% Sm. The trend is parallel to the outcome of another
similar Mg-RE research. It also has been reported by (Guan
et
al., 2018) that the addition of Sm is effective for grain
refinement of ZK60 alloy (Mg-6.0Zn-0.5Zr) due to the secondary phase containing
Sm that restricts grain growth. The mechanism behind the grain refinement
effect of rare earth on magnesium alloy is that RE acts as nucleation sites for
recrystallization and grain refinement (Ding-qian,
2008). Additionally, the formation of high melting-point Mg-RE phases
can act as the heterogeneous nucleation cores of the matrix and refine the
grain size (Zhang, Wang, and Li, 2017).
Figure 5 Effect of Sm addition on the average grain size of
ZRE1 magnesium alloy
The effect of Sm addition on the volume fraction of ZRE1 alloy has been
shown in Figure 6, where the addition of Sm shows a significant effect on ZRE1
volume fraction. The addition of Sm increased the volume fraction of Mg alloy
from 14.30 % for the base alloy to 17.64 % for 0.5 wt. % Sm addition, 19.28 %
for 1.0 wt. % Sm, 20.59 % for 1.5 wt. % Sm and 22.77 % for 2.0 wt. % Sm
addition. The trend is parallel to the outcome of other similar Mg-RE research (Ahmad et al., 2017b; Wang et al., 2014).
The study conducted by Ryou et al. (2018) examines
the correlation between grain size and grain boundary volume fraction. The
findings of the investigation indicate that a reduction in grain size leads to
an increase in the proportion of grain boundaries, owing to alterations in the
structure, including a rise in the volume fraction of triple junctions.
Figure 6 Effect of Sm addition on the volume fraction of ZRE1 magnesium alloy
Figure 6 shows the volume
fraction of the intermetallic compounds growing as a result of the addition of
Sm to the ZRE1 alloy. At the same time, the continuous networks of eutectic
compounds surround the primary grains where have a tendency to become
separated from the grains when it's refined (Sheggaf
et
al., 2017). Furthermore, the grain boundary width becomes
thicker as the content of Sm increases because Sm has a tendency to combine
with the secondary phases. It is supported by the research of (Guan et al., 2018) that the addition of Sm
increases the volume fraction of secondary intermetallic phases of ZK60 alloy.
Figure 7 (a) shows SEM
micrograph and EDS microanalyses of ZRE1 base alloy and ZRE1 alloy with 2.0
wt.% Sm addition shown in (b). SEM observations for base alloy microstructure
in (a-i) consist of grains, marked A, while surrounded by a secondary phase
crystallized along the grain boundaries like a vast morphology marked B. The
EDS spectrum of base alloy shown in (a-ii) is indicated by points consistently
showing the composition of the
matrix at region marked A and the secondary
phase of the base alloy at region B. The base alloy matrix phase comprises
magnesium, zinc, and a small amount of zirconium, while the secondary
intermetallic phase contains magnesium, zinc, and cerium.
SEM image of
ZRE1 with 2.0 wt.% Sm alloy shown in (b-i) exposed a composition of the matrix at region marked A, and two types of intermetallic phases (marked B and
C) crystallized along the grain boundaries. The EDS result in (b-ii) shows that
the intermetallic phase at point B was Mg-Zn-Ce-Sm while Mg-Zn-Sm formed at
point C. The addition of more than 2.0 wt.% Sm to base alloy shows new Mg-Zn-Sm
intermetallic compound formed at the grain boundary, where Sm forms its own
intermetallic compound out of Mg-Zn-Ce-Sm due to lack of Ce compound to form as
all intermetallic Mg-Zn-Ce-Sm (Sheggaf et al.,
2017). A previous study by (Li et al.,
2007) confirms the formation of the Mg-Zn-RE phase, where two types of RE
combined with Mg and Zn to form the Mg-Zn-RE phase. Their research adding Nd
and Y into Mg-Zn-Zr base alloy and forming Mg–Zn–Nd–Y–Zr alloys improved the
ultimate strength and elongation, as shown in Figure 8. The Mg-Zn-RE phase is
coherent with the ?-Mg matrix phase and makes the Mg-Zn-RE compound the
strengthening phase of the alloys. Incorporating rare earth elements into
magnesium alloy can lead to the creation of primary phases with high melting
points. These phases serve as nucleation cores for the
matrix and
effectively reduce the grain size through refinement. The presence of refined
grain boundaries has been observed to promote atom mobility and facilitate the
emergence of secondary metallic phases situated at the grain boundary (Zhang, Wang, and Li, 2019). Region B is where the
grain boundary is located, where the secondary intermetallic phaseforms the
Mg-Zn-Ce-Sm composition.
Figure 7 SEM micrograph and EDS microanalyses of (a)
ZRE1 base alloy and (b) ZRE1 - 2.0 wt.% Sm
The effect of Sm content addition on the tensile
properties of ZRE1Mg alloy is shown in Figure 8. The tensile test results
include the ultimate tensile strength (UTS), yield strength (YS), and
elongation (El) for the ZRE1 base alloy and the Sm-treated alloys. UTS result
shows that the addition of 0.5 wt.% Sm has a small effect on the UTS of the
alloy. The UTS only increased 3.2% from 150.3 MPa to 155.2 MPa compared to base
alloy. However, a significant increase in UTS value for 1.0 wt.% Sm at 161.7
MPa (7.5%) and 163.2 MPa (8.5%) for 1.5 wt.% Sm. This increases due to
refinement in grain size and an increase in the volume fraction of the
secondary intermetallic phase (Sheggaf et al., 2017;
Zhang et
al., 2017). While at 2.0 wt.% Sm, UTS value only slightly
increased to 156.2 MPa (3.9%) compared to base alloy due to there a decreasing
trend in grain size value (Sheggaf et al.,
2017; Zhang et al., 2017).
Figure 8 Effect of Sm content on the tensile properties of ZRE1Mg alloy
YS result shows that the
addition of 0.5 wt.% Sm has a small effect on the YS of the alloy. The YS only
increased 3.0% from 121.2 MPa to 124.9 MPa compared to base alloy. However, a
significant increase in UTS value for 1.0 wt.% Sm at 129.7 MPa (7.0%) and 130.9
MPa (7.9%) for 1.5 wt.% Sm. This increase is due to refinement in grain size
and an increase in the volume fraction of the secondary intermetallic phase (Sheggaf et al., 2017; Zhang et al.,
2017). While at 2.0 wt.% Sm, UTS value only slightly increased to 125.6 MPa
(3.6%) compared to base alloy due to there a decreasing trend in grain size
value (Sheggaf et al., 2017;
Zhang et
al., 2017). The elongation result shows that the addition
of 0.5 wt.% Sm has a small effect on the elongation of the alloy. The alloy
elongation only adds 8.1% from 4.9% elongation to 5.3% elongation compared to
the base alloy. However, significant improvement in elongation value for 1.0
wt.% Sm at 6.2% (25.9%) and 6.5% (32.3%) for 1.5 wt.% Sm. This increase is due
to refinement in grain size and an increase in the volume fraction of the
secondary intermetallic phase (Zhang et al.,
2017). While at 2.0 wt.% Sm, elongation value only slightly increased to
5.1% elongation (3.6%) compared to base alloy, due to there a decreasing trend
in grain size value (Zhang et al.,
2017).
The outcome of UTS, YS, and
elongation of this study is in line with previous research by (Zhang et al., 2017). They study the effects of
samarium addition on the microstructure and mechanical properties of
Mg-6Zn-0.4Zr magnesium alloy. They found considerable improvement in UTS and
elongation by adding 2.0 wt.% Sm. However, when the content of Sm increased
more, the UTS and elongation show declining possibly due to the MgZnSm phase
and the morphology of eutectic phases. The finding of the current study is also
consistent with those of (Li et al.,
2007) in the study on the effect of Nd and Y addition of as-cast Mg–Zn–Zr
alloy. They found out that the addition of two types of RE (Nd and Y) improved
the mechanical properties of the Mg alloy due to dendritic size refinement.
However, an increase in the amount of rare earth (RE) also resulted in a rise
in the quantity of interdimeric eutectic compounds. This formation deteriorates
the mechanical properties of Mg alloys due to their coarseness and their
location on grain boundaries. The strengthening of Mg alloys is due to a solid
solution of RE atom with borderline size and also caused by secondary phase
hardening (Sheggaf et al., 2017).
A study by Júnior et al. (2022) investigating the impact of
copper and nickel on alloys' microhardness and modulus of elasticity revealed
that the inclusion of copper resulted in a more granular microstructure,
thereby promoting a decrease in the mean value of modulus of elasticity. As per
the study mentioned earlier, the inclusion of copper led to the development of
a rougher microstructure, which consequently reduced the mean value of the
elasticity modulus. Likewise, the incorporation of samarium into the magnesium
ZRE1 alloy in the present investigation is expected to elicit analogous impacts
on the microstructural characteristics, thereby influencing the modulus of
elasticity. The observed correlation implies that the inclusion of samarium
could potentially lead to a decrease in the mean modulus of elasticity, thereby
corroborating the present study's findings.
Additionally,
the secondary intermetallic phase containing RE can deter the dislocation
movement, therefore improving the mechanical properties. However, after the
alloy achieves its peak hardness at 1.5 wt.% Sm, the hardness value trend
decreased. Where the ZRE1 alloy with 2.0 wt.% Sm shows a 57.0 HV value, a
reduction compared to the previous sample. This drop happens due to the
increase of grain size, which starts to occur when the Sm addition reaches 1.5
wt.% Sm thus reflected a reduction trend in hardness value. Previous research
by (Wang et al., 2018) also shows the same
hardness value trend. Their study on the effects of RE yttrium (Y) on Mg-Al-Zn
alloy shows that the harness value increases with the increase in Y addition,
though the trend starts moving downward after the alloy reaches its peak hardness
value. This trend is due to (i) an increase of secondary intermetallic phase
containing RE, leading to solid solution hardening effect, and (ii) grain size
coarsening of ?-Mg matrix. According to Pereira et
al. (2017), smaller grain sizes were obtained, affecting microhardness
and elongation in the alloy. Furthermore, smaller grain boundaries hinder the
movement of dislocations, resulting in enhanced resistance to deformation and
elevated microhardness values of the alloy.
The Addition of Sm
led to the formation of a new phase of MgZn(CeSm) where Ce and Sm combined to
form a secondary intermetallic phase surrounding the primary grain. The
formation of the intermetallic phase plays a major role in the improvement of
the mechanical properties of the alloy. While the identification of these
phases helped understand the strengthening mechanism of alloying treatment, Sm
addition is effective for the UTS and YS improvement. The addition of Sm leads
to intensive solution strengthening and increased dispersion strengthening of
the secondary phase, thus resulting in mechanical strength improvement. The
study shows that there is a strong relation between the microstructure and
mechanical properties of the Sm-treated ZRE1 alloy. Coarser average grain size
results in lower values of UTS, YS, elongation, and hardness. While finer
average grain size produces alloys with better UTS, YS, elongation, and
hardness. This is due to the secondary intermetallic phase containing RE, which
can deter the dislocation movement, therefore improving the mechanical
properties.
This research was supported by Universiti Tun
Hussein Onn Malaysia (UTHM) through Research Enhancement-Graduate Grant (RE-GG) (vot Q203). Communication
of this research is made possible through monetary assistance by UTHM
Publisher's Office via Publication Fund E15216. Additional support in terms of
facilities was also provided by Sustainable Manufacturing and Recycling Technology,
Advanced Manufacturing and Material Center (SMART-AMMC), Universiti Tun Hussein
Onn Malaysia (UTHM).
Adrian, M., 2012. Titanium Alloys for Aerospace Structures
and Engines A2. Introduction to Aerospace Materials, Woodhead Publishing,
pp. 202–223
Ahmad, R., Elaswad, A.M.M., Asmael, M.B.A., Shahizan,
N.R., 2017a. Effect of Yttrium Addition on Microstructure and Hardness of Cast
EV31A Magnesium Alloy. Key Engineering Materials, Volume 740, pp. 81–85
Ahmad, R., Hamzah, M.Z., Asmael, M.B.A., Sheggaf,
Z.M., 2016. Effects of Gadolinium on Microstructure and Hardness of Mg-Zn-Ce-Zr
cast alloy. ARPN Journal of Engineering and Applied Sciences, Volume 11(14),
pp. 8592–8597
Ahmad, R., Sheggaf, Z.M., Asmael, M.B.A., Hamzah,
M.Z., 2017b. Effect of Rare Earth Addition on Solidification Characteristics
and Microstructure of ZRE1 Magnesium Cast Alloy. Advances in Materials and
Processing Technologies, Volume 3(3), pp. 418–427
Al-Alimi, S., Lajis,
M.A., Shamsudin, S., 2017. Solid-State Recycling of Light Metal Reinforced
Inclusions by Equal Channel Angular Pressing: A Review. In: MATEC Web of
Conferences, Volume 135, p. 00013
Al-Alimi, S., Lajis, M.A., Shamsudin, S., Yusuf,
N.K., Chan, B.L., Didane, D.H., Rady, M.H., Sabbar, H.M., Msebawi, M.S., 2021.
Development of Hot Equal Channel Angular Processing (ECAP) Consolidation
Technique in the Production of Boron Carbide(B4C)- Reinforced Aluminium Chip
(AA6061)-Based Composite. International Journal of Renewable Energy Development,
Volume 10(3), pp. 607–621
American Society for Testing and Materials (ASTM).,
2011. Standard Guide for Preparation of Metallographic Specimens. In: American
Society for Testing and Materials (ASTM) Book of Standards
Anawati, A., Asoh, H., Ono, S., 2018. Degradation
Behavior of Coatings Formed by the Plasma Electrolytic Oxidation Technique on
AZ61 Magnesium Alloys Containing 0, 1 and 2 wt% Ca . International Journal
of Technology, Volume 9(3), pp. 622–630
American Society for Testing and Materials (ASTM)
International., 2015. Tension Testing Wrought and Cast Aluminum- and
Magnesium-Alloy Products (Metric). In: American Society for Testing and
Materials (ASTM) Book of Standards
Chauhan, A., Lohra, S., Langyan, R., 2020.
Synthesis and characterization of three ternary samarium(III) complexes and
their optical properties. Spectroscopy Letters, Volume 53, pp. 595–606
Chen, X., Li, Q., Chen, J., Zhu, L., 2019.
Microstructure and Mechanical Properties of Mg- Gd-Y-Sm-Al Alloy and Analysis
of Grain Refinement and Strengthening Mechanism. Journal of Rare Earths,
Volume 37(12), pp. 1351–1358
Chen, Y., Jin, L., Fang, D., Song, Y., Ye, R.,
2015. Effects of Calcium, Samarium Addition on Microstructure and Mechanical Properties
of AZ61 Magnesium Alloy. Journal of Rare Earths, Volume 33(1), pp. 86–92
Ding-qian, D., 2008. Effect of Rare-earth La on Microstructure
of Mg-4.5%Zn as-cast Magnesium Alloy. Light Alloy Fabrication Technology,
Volume 36(10), pp. 11–13
Enghag, P., 2004. Encyclopedia of the Elements?: Technical
Data, History, Processing, Applications. In: Wiley-VCH
Ferro, R., Saccone, A., Delfino, S., 2013.
Magnesium Alloys of the Rare Earth Metals: Systematics and Properties. Metallurgical
Science and Tecnology, Volume 16(1)
Geels, K., Fowler, D.B., Kopp, W.U., Rückert, M.,
2007. Metallographic and Materialographic Specimen Preparation, Light
Microscopy, Image Analysis and Hardness Testing. In: West Conshohocken: ASTM
international
Guan, K., Li, B., Yang, Q., Qiu, X., Tian, Z.,
Zhang, D., Zhang, D., Niu, X., Sun, W., Liu, X., Meng, J., 2018. Effects of 1.5
wt% samarium (Sm) addition on microstructures and tensile properties of a
Mg?6.0Zn?0.5Zr alloy. Journal of Alloys and Compounds, Volume 735, pp. 1737–1749
Hashmi, J.Z., Siraj, K., Latif, A., Naseem, S.,
Murray, M., Jose, G., 2019. The Role of Samarium Incorporated Structural Defects
in ZnO Thin Films Prepared by Femtosecond Pulsed Laser Deposition. Journal
of Alloys and Compounds, Volume 800, pp. 191–197
Huang, Z.H., Liang, S.M., Chen, R.S., Han, E.H.,
2009. Solidification Pathways and Constituent Phases of Mg-Zn-Y-Zr Alloys. Journal
of Alloys and Compounds, Volume 468(1–2), pp. 170–178
Institute of Rare Earths and Strategic Metals eV.,
2020. Rare Earth Prices | Institute of Rare Earths and Strategic Metals eV. Available
Online at: https://en.institut-seltene-erden.de/our-service-2/Metal-prices/rare-earth-prices/,
Accessed on 01/14/2020)
Jahedi, M., McWilliams, B.A., Knezevic, M., 2018.
Deformation and Fracture Mechanisms in WE43 Magnesium-rare Earth Alloy Fabricated
by Direct-chill Casting and Rolling. Materials Science and Engineering A,
Volume 726(March), pp. 194–207
Júnior, P. F., De Olivé Ferreira, L., Garcao, W.
J. L., De Paula Almeida, R., Melo, C. M., & Ferreira, A. F. (2022).
Heat-flow parameters affecting microstructure and mechanical properties of
Al-Cu and Al-Ni alloys in directional solidification: An experimental
comparative study. International Journal of Materials Research, 113(3),
181–193
Ke-jie, L., Quan-an, L., Xiao-tian, J., Jun, C.,
Xing-yuan, Z., Qing, Z., 2009. Effects of Sm Addition on Microstructure and Mechanical
Properties of Mg-6Al-0.6Zn Alloy. Scripta Materialia, Volume 60(12), pp.
1101–1104
Kiani, M., Gandikota, I., Rais-rohani, M., Motoyama,
K., 2017. Design of Lightweight Magnesium Car Body Structure Under Crash and Vibration
Constraints. Journal of Magnesium and Alloys, Volume 2(2), pp. 99–108
Kirman, Zulfia, A., Suharno, B., 2016. Effects of
Magnesium on Properties of AlZrCe-Mg-Al2O3 Nanocomposites. International
Journal of Technology, Volume 7(3), pp. 447–455
Kristanto, G.A., Gusniani, I., Ratna, A., 2015.
The performance of municipal solid waste recycling program in Depok, Indonesia.
International Journal of Technology, Volume 6(2), pp. 264–272
Kusrini, E., Ayuningtyas, K., Mawarni, D.P.,
Wilson, L.D., Sufyan, M., Rahman, A., Prasetyanto, Y.E.A., Usman, A., 2021.
Micro-structured Materials for the Removal of Heavy Metals using a Natural
Polymer Composite. International Journal of Technology, Volume 12(2), pp.
275–286
Li, Q., Wang, Q., Wang, Y., Zeng, X., Ding, W.,
2007. Effect of Nd and Y Addition on Microstructure and Mechanical Properties
of as-cast Mg-Zn-Zr alloy. Journal of Alloys and Compounds, Volume 427(1–2),
pp. 115–123
Li, K., Xu, H., Tong, Z., 2019. Effects of Sm on
Microstructure of Mg-12Gd-2Y-0.5Zr Alloy. In: IOP Conference Series:
Materials Science and Engineering, Volume 569(2), p. 022017
Liu, L., Chen, X., Pan, F., Gao, S., Zhao, C.,
2016. A New High-strength Mg-Zn-Ce-Y-Zr Magnesium Alloy. Journal of Alloys
and Compounds, Volume 688, pp. 537–541
Liu, B.S., Wang, H.H., Zhang, Y.Z., Yang, Y.X.,
Ren, X.X., Du, H.Y., Hou, L.F., Wei, Y.H., Song, G.L., 2021. The influence of Adding
Samarium on the Microstructure, Mechanical Performance and Corrosion Behavior
of as-extruded AZ41 Alloys. Journal of Physics and Chemistry of Solids, Volume
150, p. 109851.
Lucas, J., Lucas, P., Le-Mercier, T., Rollat, A.,
Davenport, W., 2014. Rare Earths Rare Earths Production and Use. In: Elsevier
B.V.
Luo, Q.Y., Luo, H., Kuang, H.M., Chen, W.T., Wen,
Y.X., 2019. A novel samarium material: Synthesis, structure, photophysical
properties and photoluminescence energy transfer mechanism. Journal of Solid
State Chemistry, Volume 270, pp. 200–204
Marzouk, S.Y., Hammad, A.H., 2020. Influence of Samarium
Ions on the Structural, and Optical Properties of Unconventional Bismuth Glass Analyzed
using the Judd–Ofelt Theory. Journal of Luminescence, Volume 231, p.
117772
Materialstechnology., 2019. Weblinks : Magnesium
Applications. TMS. Available Online at: www.materialstechnology.org, Accessed
on 03/03/2021
Moosbrugger, C., 2017. Engineering Properties of
Magnesium Alloys. In: ASM International (Issue M)
Pereira, P.H.R., Huang, Y., Langdon,
T.G., 2017. Thermal Stability and Superplastic
Behaviour Of An Al-Mg-Sc Alloy Processed by ECAP And HPT At Different
Temperatures. In: IOP Conference Series: Materials Science and
Engineering
Purnama, D., Winarto, W., Sofyan, N., Prihastomo,
A., Ito, K., 2020. Microstructure and Mechanical Properties of Ah-36 Steel
Weldment Welded using Magnesium Modified E6013 Electrode. International
Journal of Technology. Volume 11(1), pp. 48–59
Rady, M.H., Mahdi, A.S., Mustapa, M.S.,
Shamsudin, S., Lajis, M.A., Msebawi, M.S., Siswanto, W.A., Al Alimi, S., 2019.
Effect of Heat Treatment on Tensile Strength of Direct Recycled Aluminium Alloy
(AA6061). Materials Science Forum, Volume 961, pp. 80–87
Rogal, L., Kania, A., Berent, K., Janus, K.,
Litynska-Dobrzynska, L., 2019. Microstructure and Mechanical Properties of
Mg-Zn-RE-Zr Alloy After Thixoforming. Journal of Materials Research and
Technology, Volume 8(1), pp. 1121–1131
Rokhlin, L.L., Dobatkina, T.V., Tarytina, I.E.,
Luk’yanova, E.A., Temralieva, D.R., 2021. Effect of Samarium on the Recovery in
Gadolinium-Containing Magnesium Alloys. Russian Metallurgy (Metally), Volume
2021(7), pp. 816–820
Ryou, H., Drazin, J.W., Wahl, K.J., Qadri, S.B.,
Gorzkowski, E.P., Feigelson, B.N., Wollmershauser, J.A., 2018. Below the
Hall–petch Limit In Nanocrystalline Ceramics. American Chemical Society (ACS ) Nano,
Volume 4(12), pp. 3083–3094
Rzychon, T., Kie?bus, A., Szala, J., 2008.
Quantitative Description of Microstructure of ZRE1 Magnesium Alloy. Inzynieria
Materialowa, Volume 29(May), pp. 299–303
Sadeq, M.S., Morshidy, H., 2020. Effect of Samarium
Oxide on Structural, Optical and Electrical Properties of Some Alumino-borate Glasses
with Constant Copper Chloride. Journal of Rare Earths, Volume 38, pp. 770–775
Sheggaf, Z.M., Ahmad, R., Asmael, M.B.A., Elaswad,
A.M.M., 2017. Solidification, microstructure, and mechanical properties of the
as-cast ZRE1 magnesium alloy with different praseodymium contents. International
Journal of Minerals, Metallurgy and Materials, Volume 24, pp. 1306–1320
StJohn, D.H., Qian, M., Easton, MA., Cao, P., Hildebrand,
Z., 2005. Grain refinement of magnesium alloys. Metallurgical and Materials
Transactions A, Volume 36(7), 1669–1679
Tekumalla, S., Seetharaman, S., Almajid, A.,
Gupta, M., 2014. Mechanical Properties of Magnesium-rare Earth Alloy Systems: A
Review. Metals, Volume 5(1), pp. 1–39
Wang, M.F., Xiao, D.H., Zhou, P.F., Liu, W.S.,
Ma, Y.Z., Sun, B.R., 2018. Effects of rare earth yttrium on microstructure and
properties of Mg–Al–Zn alloy. Journal of Alloys and Compounds, Volume 742,
pp. 232–239
Wang, Y., Wu, G., Liu, W., Pang, S., Zhang, Y.,
Ding, W., 2014. Effects of Chemical Composition on the Microstructure and Mechanical
Properties of Gravity Cast Mg-xZn- yRE-Zr alloy. Materials Science and
Engineering A, Volume 594, pp. 52–61
Yang, Z., Li, J.P., Zhang, J.X., Lorimer, G.W.,
Robson, J., 2008. Review on Research and Development of Magnesium Alloys. Acta
Metallurgica Sinica, Volume 21(5), pp. 313–328
Yunwei, G., Quanan, L., Xiaoya, C., Zhitao, L.,
2018. As-cast Microstructures and Mechanical Properties of Mg-5Y-2Nd-xSm-0.5Zr
(x = 0, 1, 3, 5). Magnesium Alloys, Volume 7(2), p. 58
Zhang, Q., Wang, Y., Li, P., 2019. Grain-refinement Strengthening Effect of Y and Sm on Magnesium Alloy AZ81. In: IOP Conference Series: Materials Science and Engineering, Volume 592(1), p. 012027
Zhang, Y., Huang, X., Li, Y., Ma, Z., Ma, Y., Hao, Y., 2017. Effects of Samarium Addition on as-cast Microstructure, Grain Refinement and Mechanical Properties of Mg-6Zn- 0.4Zr Magnesium Alloy. Journal of Rare Earths, Volume 35(5), pp. 494–502
