Catalytic Cracking of Palm Fatty Acid Distillate with NaOH and KOH Catalyst Supported by Gamma Alumina
Corresponding email: rismawati.rasyid@umi.ac.id
Published at : 17 May 2024
Volume : IJtech
Vol 15, No 3 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i3.5980
Rasyid, R., Prasetyo, D., Fitriani, N., Syarif, T., 2024. Catalytic Cracking of Palm Fatty Acid Distillate with NaOH and KOH Catalyst Supported by Gamma Alumina. International Journal of Technology. Volume 15(3), pp. 770-779
| Rismawati Rasyid | Departement of Chemical Engineering, Faculty of Industrial Technology, Universitas Muslim Indonesia, Makassar, Indonesia |
| Dicky Prasetyo | Departement of Chemical Engineering, Faculty of Industrial Technology, Universitas Muslim Indonesia, Makassar, Indonesia |
| Nurul Fitriani | Departement of Chemical Engineering, Faculty of Industrial Technology, Universitas Muslim Indonesia, Makassar, Indonesia |
| Takdir Syarif | Departement of Chemical Engineering, Faculty of Industrial Technology, Universitas Muslim Indonesia, Makassar, Indonesia |
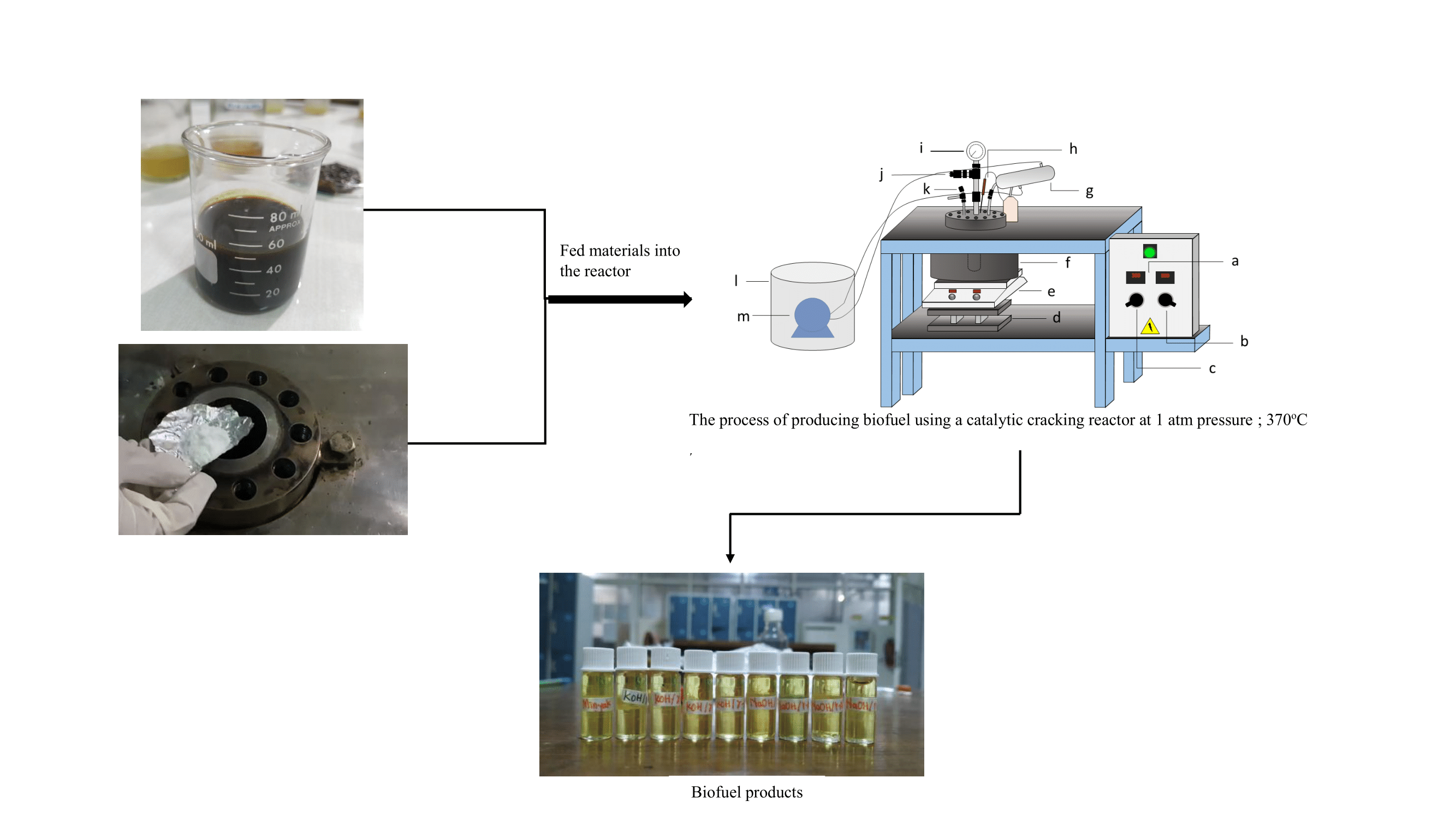
This article describe about the study of produce fuel from
palm fatty acid distillate with a similar composition to
fossil fuels through catalytic
cracking method using alkaline heterogeneous catalyst. The catalytic cracking reaction was operated at batch reactor with a
constant temperature of 370oC, a volume of 50 mL of feedstock, a
pressure of 1 atm, and two
kinds of catalysts: NaOH/g-Al2O3
and KOH/g-Al2O3 which
has been characterization with X-ray diffraction and scanning electron
microscopy. The best catalyst to produce biofuel type biogasoline (C5-C15)
is KOH/g-Al2O3 (5%) with a
yield of 70% and selectivity to biogasoline of 74.46%. Meanwhile, the best
catalyst to produce biofuel type biodiesel (C15-C22) is NaOH/g-Al2O3 (5%) with a
yield of 80% and selectivity to biodiesel of 67.72%.
Biodiesel; Biogasoline; Catalytic Cracking; Palm fatty acid distillate
The depletion of fossil fuels, coupled with the rapid growth in living
standards, has led to a significant increase in fuel prices and the subsequent
impact on greenhouse gas emissions. This has prompted researchers to prioritize
the development of renewable and non-polluting fuels, which hold the promise of
achieving global energy security while also mitigating the effects of climate
change (Ibrahim, et al., 2020; Singh et al., 2020; Wakoc, et al., 2018). Palm
oils are widely considered to be the most promising feedstock for the
production of such alternative fuels, known colloquially as biofuels.
Nevertheless, if palm oil is used as a raw resource for biofuels for an
extended period, it will eventually compete with foodstuffs. Using byproducts
of palm oil processing, which still contain a lot of fatty acids as a raw
material is a very wise decision (Oliveira, et
al., 2021; Arita et al., 2020; Onlamnao and Tippayawong, 2020; Zaher, et
al., 2017).
Among all biofuel preparation technologies, catalytic cracking is considered a promising method due to its simple process which can be carried out at atmospheric pressure, has a higher cracking conversion efficiency, higher light alkene selectivity, less carbon deposition and the production of several types of liquid products similar to petroleum-based fuels. Furthermore, with appropriate catalysts, catalytic cracking can be used to produce gasoline, kerosene, and diesel, and it has good compatibility with various feedstocks as well as a lower cost (Ulfiati et al., 2022; Zhang, et al., 2021; Orazbayev, et al., 2020; Nieuwelink et al., 2020; Singh et al., 2020; Mammadova et al., 2018). The cracking reaction is characterized primarily by a break in the "carbon-carbon" bond, indicating an endothermic reaction—the higher the temperature, the faster the reaction (Wang et al., 2019). In comparison to the transesterification process, the catalytic cracking process is a more efficient method that offers greater flexibility in product output. The transesterification process, on the other hand, involves several complex steps, including the separation of products and by-products and the initial treatment of high FFA to prevent saponification reactions. Additionally, this process produces only one type of biofuel, and the production time can be relatively long, with processing times of around 5 hours and purification times of up to 24 hours (Wahyono et al., 2022; Rasyid et al., 2018). Meanwhile, when compared to the hydrocracking process, which requires high temperatures 350oC and 29.6077 atm pressures, the catalytic cracking process is more cost-effective because it can be operated at lower temperatures and atmospheric pressure (Trisunaryanti et al., 2020; Al-Muttaqi et al., 2019; Rasyid et al., 2015).
Currently, to meet the requirements of vehicle fuel
standards, catalytic cracking must reduce the acid value and oxygen content of
the biofuel which are biogasoline (C5-C15) dan
biodiesel (C16-C22) to improve fuel
properties such as density, calorific value, dynamic viscosity, and other
parameters (Sardi et al., 2022; Makertihartha et al., 2020). There is no doubt that
catalysts play an important role in the catalytic cracking of fatty
acids to produce biofuel. Alkaline catalysts
such as sodium hydroxide (NaOH) and potassium hydroxide (KOH) are commonly used
in the production of biofuels because their rate of reaction is much faster
than that of acid catalysts (Min et al., 2015).
High catalytic activity, shorter reaction time, simple operating
conditions, low cost, and availability are just a few of the advantages of NaOH
and KOH catalysts. However, homogeneous base catalysts, on the other hand, are
extremely sensitive to free fatty acids and water. Therefore, a homogeneous
catalyst was modified into a heterogeneous catalyst by the addition of a
catalyst support (Widayat et al.,
2016).
Heterogeneous catalysts can be designed to graft and trap
active molecules on the surface or within the pores of solid supports such as
silica, alumina, or CaO (Thangaraj et
al., 2019). Gamma alumina (-Al2O3) is used as a catalyst support because it has a large surface area
(150-300 m2/g) and an amphoteric acid and base active site with
various strengths depending on the method of production. Furthermore,
-Al2O3 serves the primary function of providing surface area for the active
component, with the purpose of increasing contact between the active core and
the reactants while maintaining active phase activity (Rasyid et al., 2018). The presence of
acid sites on Al2O3, which are associated with Lewis
acidity and very weak Brönsted acidity, makes it an effective catalyst for
deoxygenation with minimal aromatization (Istadi et
al., 2021). Thus, making heterogeneous NaOH/g-Al2O3
and KOH/g-Al2O3
catalysts may be a feasible method to integrate the required acidic and basic
characteristics to overcome defects in NaOH and KOH catalysts and avoid an
excessive production of aromatic hydrocarbons by acid solid catalysts (Zheng et al.,
2019).
Therefore, the
catalytic cracking of palm fatty acid distillate for biofuels was carried out
using heterogeneous NaOH/-Al2O3
and KOH/
-Al2O3
catalysts. Mechanically, NaOH/g-Al2O3
and KOH/g-Al2O3
catalysts were created by combining NaOH and KOH as primary catalysts and
-Al2O3
as catalysts support. This research aims to produce a fuel with a similar
composition to fossil fuels.
2.1. Tools
2.2. Preparation of Catalysts
The catalyst was prepared from sodium hydroxide (NaOH) p.a
merck and potassium hydorxide (KOH) p.a merck as catalyst site active element which impragnated into
support gamma alumina (g-Al2O3) from merck (p.a). Wet
impregnation was used to prepare the catalysts. NaOH 0.5 N was impregnated into 10
grams of g-Al2O3 to
prepare NaOH/g-Al2O3 catalyst, then it was stirred with a magnetic
hotplate stirrer while the water in the mixture evaporated to form a paste. The mixture of the catalysts would be dried at 110oC within 8 hours. Afterward, the mixture was calcined
for 3 hours at a temperature of 500oC. Furthermore, the same process was carried out with
KOH 0.5 N to preparation KOH/g-Al2O3
catalyst.
2.3. Catalytic
Cracking Process
A batch reactor
with a pressure of 1 atm was used to conduct the reaction. The reactor is
filled with 50 mL of palm fatty acid distillate from palm oil
refining, 0.5 grams of NaOH/-Al2O3
catalyst (1% of the raw ingredients), and a
magnetic stirrer. The reaction is then performed for two hours after the
reactor heater is turned on until it reaches a temperature of 370°C (Aziz et al., 2020). The biofuel product
will evaporate from the reactor to the liquid product container during the
reaction and flow through the condenser. Remaining in the reactor is the
residue, and the amount of gaseous product that hasn't condensed is estimated
using the mass balance equation by deducting the initial amount of raw material
from the final product. Furthermore, the catalytic cracking
process is carried out with a NaOH/
-Al2O3
catalyst (3, 5, 7)% and KOH/
-Al2O3
catalyst (1, 3, 5, 7)%.
·
Scanning Electron
Microscopy (SEM) and X-Ray Diffraction (XRD) at the following conditions: 40
KV, 15 mA, CuK/1.54060 Time/step of 23.9700 s, step size of 0.0220 deg, and
Scan axis Gonio were used to characterize the catalyst.
· Gas chromatography-mass spectrometry (GC-MS) with an Agilent capillary number of 19.091 S-493, HP-5MS of 5% phenyl methyl siloxane, nominal length of 30.0 m, nominal diameter of 250 um, nominal film thickness of 0.25 um, and nominal initial pressure of 10.5 psi was used to analyze the product's component compounds.
3.1. Characterization of NaOH/Al2O3 and KOH/
Al2O3 Catalyst
3.1.1 X-Ray Diffraction (XRD) Analysis
X-ray Diffraction (XRD) was used to identify content that was impregnated on the support of catalyst (Al2O3) as the NaOH/
Al2O3 and KOH/
Al2O3 catalysts (Figure 2).
Figure 2 XRD diffractogram (a) NaOH/Al2O3 catalyst, and (b) KOH/
Al2O3 catalysts
According to ICDD (International Center for Diffraction Data) 00-010-0425, Gamma Alumina (g-Al2O3) has peaks at 2? = 37°, 39°, 45°, and 67°. As shown in Figure 2, NaOH/g-Al2O3 has peaks 2? that are similar to g-Al2O3, such as 37o, 39o, and 67o, but there are new peaks formed due to impregnation, indicating the presence of deposited NaOH catalyst. Likewise, the KOH/Al2O3 catalyst has peaks 2? that are similar to
Al2O3, notably 37o, 39o, and 67o, and there are new peaks are formed due to impregnation, proving the presence of deposited KOH catalyst (Singh et al., 2020; Yu et al., 2019; Wako et al., 2018).
3.1.2 Scanning Electron Microscopy (SEM) Analysis

The surface morphology of the catalyst support revealed a regular crystal structure before impregnation, whereas after impregnation revealed that the impregnated active site had attached and distributed to the support's main structure.
3.2. Gas Chromatography-Mass Spectrometry Analysis of Palm Fatty Acid Distillate
Palm fatty acid distillate was GC-MS analyzed before the catalytic cracking process to determine the compound composition of the raw material used (Table 1). The by-product of palm oil processing used in this study still contains triglyceride components, specifically free fatty acids. As noted by (Oliveira et al., 2021), the conversion of palm oil into cooking oil can result in up to 6% of the by-product of feed CPO being left behind. The byproduct is palm oil fatty acid distillate, which contains a high concentration of free fatty acids. There are also significant amounts of hydrocarbon compounds (Table 1). This shows that palm oil waste has the potential to be used as a raw material in the production of biofuels.
3.3. Product Yield Analysis Results
The yield of the product obtained in the biofuel production process by catalytic cracking of palm fatty acid distillate with NaOH/Al2O3 and KOH/
Al2O3 catalysts was directly proportional to the catalyst concentration up to 5% and experienced product yields at 7% catalyst concentration (Figure 4). Studies by (Thambiyapillaia and Ramanujam, 2021; Akah, Williams, and Ghrami, 2019; Prabasari, et al., 2019) have demonstrated that the addition of a catalyst to a catalytic cracking reaction can increase the reaction rate, resulting in higher yields. However, it is important to note that if the catalyst's performance has already reached its optimum limit, adding more of it beyond that point will not lead to further improvements in the reaction's effectiveness.
Despite the similarities in the line chart, the amount of yield produced by each catalyst is different. The yield obtained with the NaOH/Al2O3 catalyst was greater than the yield obtained with the KOH/
Al2O3 catalyst. The product yield obtained with the NaOH/g-Al2O3 (5%) catalyst was 80% while the higher yield of KOH/g-Al2O3 (5%) was 70%. This is due to the active site distribution of the NaOH/
Al2O3 catalyst obtained through scanning electron microscopy analysis appearing wider and adhering more to the catalyst support (Figure 3).
3.3. Selectivity Product
The highest conversion of biodiesel product from the catalytic cracking of palm fatty acid distillate was obtained with NaOH/g-Al2O3 (5%) catalyst, which was 67.72%. This shows that the NaOH/-Al2O3 catalyst is more selective towards long-chain biofuels (C15-C-22) compared to the KOH/g-Al2O3 catalyst, which produces less than 20% biodiesel.
Meanwhile, the conversion of biogasoline products from the catalytic cracking of palm fatty acid distillates with the NaOH/Al2O3 catalyst shows data that is directly proportional to the increase in the catalyst. The highest biogasoline product with NaOH/
Al2O3 catalyst was obtained at 7% catalyst, which was 48.88%, indicating that if the catalyst is increased again, the conversion may increase or decrease. However, when compared to the catalytic cracking of palm fatty acid distillate with a KOH/
Al2O3 catalyst, better results were obtained, where the optimum biogasoline production point was obtained with a KOH/
Al2O3 catalyst (5%) and a bio gasoline yield of 74.46%. This shows that the KOH/
Al2O3 catalyst is more selective towards short-chain biofuels (C5-C15) than the NaOH/
Al2O3 catalyst. According to (Aziz et al., 2021b; Istadi et al., 2021; Senter et al., 2021), catalysts that produce short-chain biofuels have high performance. The product's selectivity is proportional to the Lewis to Brønsted ratio (L/B ratio). When the L/B ratio is high, the Lewis acid site is dominant. Because of the catalyst's low L/B ratio, the NaOH/
Al2O3 catalyst promotes the formation of long chains (biodiesel).
Palm fatty acid distillate (PFAD) can be converted into biogasoline (C5–C15) and biodiesel (C16–C22) at a pressure of 1 atm using NaOH/Al2O3 and KOH/
Al2O3 as catalysts in a catalytic cracking process. KOH/
Al2O3 (5%) is the best catalyst for producing biofuel type biogasoline (C5-C15), with a yield of 70% and a selectivity to biogasoline of 74.46%. Meanwhile, the best catalyst for producing biofuel type biodiesel (C15-C22) is NaOH/
Al2O3 (5%), which has an 80% yield and a 67.72% selectivity for biodiesel. Furthermore, the product of this research can be utilized as a blend of commercial fuels, given that they contain the same compounds and that the combustion products are easily decomposed, minimizing pollution to the environment. It is also intended to reduce the use of fossil fuels, ensuring global energy availability. This research can be expanded with different pre-treatments in the manufacture of catalysts to increase their effectiveness, as well as the addition of appropriate promoters.
DRPM KEMENDIKBUDRISTEK for assistance with
funding in the PTUPT research scheme (Number : 2327.I/B.07/UMI/VII/2022). The
academic community of Fakultas Teknologi Industri Universitas Muslim Indonesia,
where the research is held at the Chemical Engineering Process Laboratory.
Akah,
A., Williams, J., Ghrami, M., 2019. An Overview of Light Olefins Production via
Steam Enhanced Catalytic Cracking. Catalysis Surveys from Asia, Volume
23, pp. 265–276
Al-Muttaqi,
M., Kurniawansyah, F., Prajitno, D.H., Roesyadi, A., 2019. Hydrocarbon Biofuel
Production by Hydrocracking Process with Nickel-Iron Supported on HZSM-5
Catalyst. In: IOP Conference Series: Materials Science and Engineering,
Volume 543(1), p. 012055
Arita, S., Nazarudin, N., Rosmawati, R., Komariah, L.N.,
Alfernando, O., 2020. The
Effect of Combined H-USY and ZSM-5 Catalyst in Catalytic Cracking of Waste
Cooking Oil to Produce Biofuel. In: AIP Conference Proceedings, Volume
2242(1), p. 040047
Aziz,
I., Ardine, E.A.F., Saridewi, N., Adhani, L., 2021a. Catalytic Cracking of
Crude Biodiesel into Biohydrocarbon Using Natural Zeolite Impregnated Nickel
Oxide Catalyst. Jurnal Kimia Sains dan Aplikasi, Volume 24(7), pp. 222–227
Aziz, I., Kurnianti, Y., Saridewi, N., Adhani, L.,
Permata, W., 2020. Utilization
of Coconut Shell as Cr2O3 Catalyst Support for Catalytic
Cracking of Jatropha Oil into Biofuel. Jurnal Kimia Sains dan Aplikasi, Volume
23(2), pp. 39–45
Aziz,
I., Retnaningsih, T., Gustama, D., Saridewi, N., Adhani, L., Dwiatmoko, A.A.,
2021b. Catalytic Cracking of Jatropha Oil into Biofuel over Hierarchical
Zeolite Supported NiMo Catalyst. In: AIP Conference Proceedings, Volume
2349(1), p. 020004
Ibarra,
Á., Hita, I., Azkoiti, M.J., Arandes, J.M., Bilbao, J., 2019. Catalytic
Cracking of Raw Bio-oil under FCC Unit Conditions over Different Zeolite-based
Catalysts. Journal of Industrial and Engineering Chemistry, Volume 78,
pp. 372–382
Ibrahim,
H., Silitonga, A.S., Rahmawaty, Dharma, S., Sebayang, A.H., Khairil.,
Sumartono, Sutrisno, J., Razak, A., 2020. An Ultrasound Assisted
Transesterification to Optimize Biodiesel Production from Rice Bran Oil. International
Journal of Technology, Volume 11(2), pp. 225–234
Istadi,
I., Riyanto, T., Buchori, L., Anggoro, D.D., Pakpahan, A.W., Pakpahan, A.J.,
2021. Biofuels Production from Catalytic Cracking of Palm Oil Using Modified HY
Zeolite Catalysts over A Continuous Fixed Bed Catalytic Reactor. International
Journal of Renewable Energy Development, Volume 10(1), pp. 149–156
Makertihartha,
I.G.B., Fitradi, R.B., Ramadhani, A.R., Laniwati, M., Muraza, O., Subagjo,
2020. Biogasoline Production from Palm Oil: Optimization of Catalytic Cracking
Parameters. Arabian Journal for Science and Engineering, Volume 45, pp.
7257–7266
Mammadova,
T., Abbasov, M., Movsumov, N., Latifova, T., Hasanova, A., Kocharli, Z., Irada,
K., Abbasov, V., 2018.
Production of Diesel Fractions by Catalytic Cracking of Vacuum Gas Oil and Its
mixture with Cottonseed Oil under the influence of a Magnetic Field, Egyptian
Journal of Petroleum, Volume 17, pp. 1029–1033
Min,
P.H., Shahbaz, K., Rashmi, W., Mjalli, F.S., Hashim, M.A., Alnashef, I.M.,
2015. Removal of Residual Calatyst from Palm Oil-based Biodiesel Using New
Ionic Liquids Analogous. Journal of Engineering Science and Technology,
Volume 4(4), pp. 35–49
Nieuwelink,
A.E., Velthoen, M.E., Nederstigt, Y.C., Jagtenberg, K.L., Meirer, F.,
Weckhuysen, B.M., 2020. Single Particle Assay to Determine Heterogeneities
within Fluid Catalytic Cracking Catalysts. Chemistry-A European Journal,
Volume 26, pp. 8564– 8554
Oliveira,
B.F.H.d., de-França, L.F., Fernandes-Corrêa, N.C., Ribeiro, N.F.D.P.,
Velasquez, M., 2021. Renewable Diesel Production from Palm Fatty Acids
Distillate (PFAD) via Deoxygenation Reactions. Catalysts, Volume 11(9),
pp. 1–16
Onlamnao,
K., Tippayawong, N., 2020. Organic Liquid Products from Cracking of Used
Cooking Oils with Commercial Catalysts. Chemical Engineering Transactions,
Volume 78, pp. 55–60
Orazbayev,
B., Kozhakhmetova, D., Wójtowicz, R. Krawczyk, J., 2020. Modeling of a
Catalytic Cracking in the Gasoline. Energies, Volume 13(18), pp. 1–13
Prabasari,
I.G., Sarip, R., Rahmayani, S., Nazarudin, 2019. Catalytic Cracking of Used
Cooking Oil Using Cobalt-impregnated Carbon Catalysts. Makara Journal of
Science, Volume 23(3), pp. 162–168
Rasyid,
R., Prihartantyo, A., Mahfud, M., Roesyadi, A., 2015. Hydrocracking of
Calophyllum inophyllum Oil with Non- Sulfide CoMo Catalysts. Bulletin of
Chemical Reaction Engineering and Catalysis, Volume 10(1), pp. 61–69
Rasyid, R., Sabara, Z., Ainun-Pratiwi, H., Juradin,
R., Malik, R., 2018. The
Production of Biodiesel from A Traditional Coconut Oil Using NaOH/?-Al2O3
Heterogeneous Catalyst. Makassar. In: IOP Conference Series: Earth and
Environmental Science, Volume 175, p. 012025
Sardi,
B., Ningrum, R.F., Ardianyah, V.A., Qadariyah, L., Mahfud, M., 2022. Production
of Liquid Biofuels from Microalgae Chlorella sp. via Catalytic Slow Pyrolysis. International
Journal of Technology, Volume 13(1), pp. 147–156
Senter,
C., Mastry, M.C., Zhang, C.C., Maximuck, W.J., Gladysz, J.A., & Yilmaz, B.,
2021. Role of Chlorides in Reactivation of Contaminant Nickel on Fluid
Catalytic Cracking (FCC) Catalysts. Applied Catalysis A: General, Volume
611, p. 117987
Singh, H. K. G., Yusup, S., Quitain, A. T., Abdullah,
B., Ameen, M., Sasaki, M., Kida, T., Cheah, K. W., 2020. Biogasoline Production from Linoleic Acid via
Catalytic Cracking over Nickel and Copper-doped ZSM-5 Catalysts. Environmental
Research, Volume 186, p. 109616
Thambiyapillaia,
S., Ramanujam, M., 2021. An Experimental Investigation and Aspen HYSYS
Simulation of Waste Polystyrene Catalytic Cracking Process for the Gasoline
Fuel Production. International Journal of Renewable Energy Development, Volume
10(4), pp. 891–900
Thangaraj,
B., Solomon, P.R., Muniyandi, B., Ranganathan, S., Lin, L., 2019. Catalysis in
Biodiesel Production. Clean Energy, Volume 3(1), pp. 2–23
Trisunaryanti,
W., Triyono. T., Ghoni, M.A., Fatmawati. D.A., Mahayuwati. P.N., Suarsih, E.,
2020. Hydrocracking of Callophyllum Inophyllum Oil Employing Co and/or Mo
Supported on g-Al2O3 for
Biofuel Production. Bulletin of Chemical Reaction Engineering &
Catalysis, Volume 15 (3), pp. 743–751
Ulfiati,
R., Dhaneswara, D., Harjanto, S., Fatriansyah, J.F., 2022. Synthesis and
Characterization ZSM-5 Based on Kaolin as a Catalyst for Catalytic Cracking of
Heavy Distillate. International Journal of Technology, Volume 13(4), pp.
860–869
Wahyono,
Y., Hadiyanto, Budihardjo, M.A., Hariyono, Y., Baihaqi, R.A., 2022.
Multifeedstock Biodiesel Production from a Blend of Five Oils through
Transesterification with Variation of Moles Ratio of Oil: Methanol. International
Journal of Technology, Volume 13(3), pp. 606–618
Wako,
F.M., Reshad, A.S., Bhalerao, M.S., Goud, V.V., 2018. Catalytic Cracking of
Waste Cooking Oil for Biofuel Production Using Zirconium Oxide Catalyst. Industrial
Crops & Products, Volume 118, pp. 282–289
Wang,
C., Tian, X., Zhao, B., Zhu, L., Li, S., 2019. Experimental Study on Spent FCC
Catalysts for the Catalytic Cracking Process of Waste Tires. Processes, Volume
7(6), p. 335
Widayat,
W., Wicaksono, A.R., Firdaus, L.H., Okvitarini, N., 2016. Synthesis H-Zeolite
Catalyst by Impregnation KI/KIO3 and Performance Test Catalyst for Biodiesel
Production Synthesis H-Zeolite Catalyst by Impregnation KI/KIO3 and Performance
Test Catalyst for Biodiesel Production. In: IOP Conference Series:
Materials Science and Engineering, Volume 107(1), p. 012044
Yu,
H., Liu, Y., Liu, J., Chen, D., 2019. High Catalytic Performance of an
Innovative Ni/Magnesium Slag Catalyst for The Syngas Production and Tar Removal
from Biomass Pyrolysis. Fuel, Volume 254, p. 115622
Zaher,
F., Gad, M.S., Aly, S.M., Hamed, S.F., Abo-Elwafa, G.A., Zahran, H.A., 2017.
Catalytic Cracking of Vegetable Oils for Producing Biofuel. Egypt Journal
Chemical, Volume 60(2), pp. 291–300
Zhang,
Y., Li, Z., Wang, Z. Ji, Q., 2021. Optimization Study on Increasing Yield and
Capacity of Fluid Catalytic Cracking (FCC) Units. Processes, Volume 9,
p. 1497
Zheng,
Z., Lei, T., Wang, J., Wei, Y., Liu, X., Yu, F., Ji, J., 2019. Catalytic
Cracking of Soybean Oil for Biofuel over ?-Al2O3/CaO Composite Catalyst. Journal
of The Brazilian Chemical Society, Volume 30(2), pp. 359–370
