Mass Transfer Phenomena during the Ultrasound-assisted Extraction of Algal Oil from Spirulina sp.
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.5974
Nisya, A.F., Rochmadi, R., Budiman, A., 2024. Mass Transfer Phenomena during the Ultrasound-assisted Extraction of Algal Oil from Spirulina sp. International Journal of Technology. Volume 15(4), pp. 927-936
| Alinda Fitrotun Nisya | 1 Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No.2 Yogyakarta, 55281, Indonesia 2 Department of Chemical Engineering, Faculty of Industrial Te |
| Rochmadi Rochmadi | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No.2 Yogyakarta, 55281, Indonesia |
| Arief Budiman | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No.2 Yogyakarta, 55281, Indonesia |
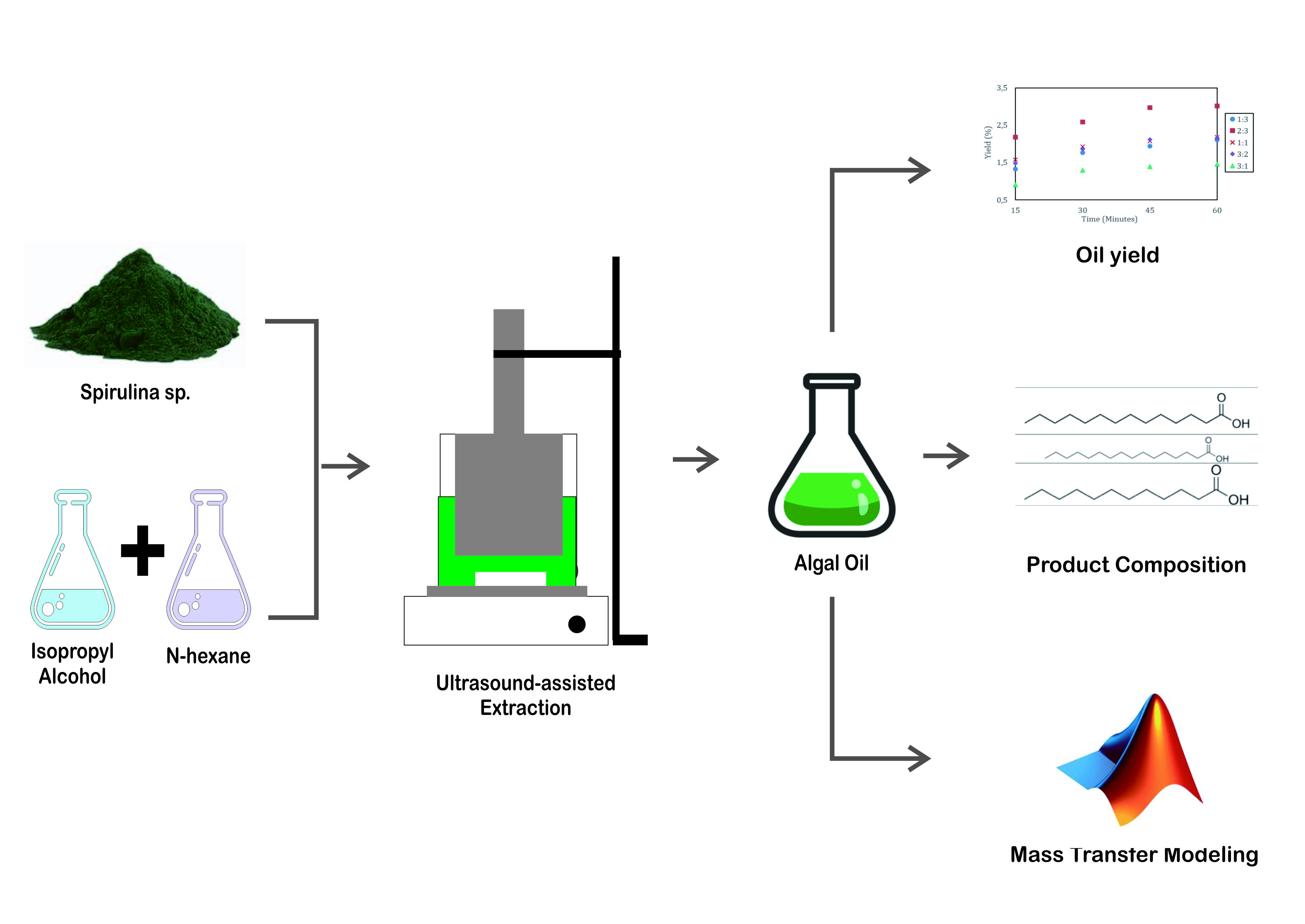
Recently, microalgae is potential to be developed because it is easy to cultivate. This study investigated the effect of the solvent ratio on the mass transfer of algal oil at various residence times from 15 minutes to 60 minutes during ultrasound-assisted extraction process at 25 kHz and stirred at 150 rpm. The highest amount of the algal oil yielded was 3.01%, obtained by a ratio of isopropyl alcohol to n-hexane of 2:3 ratio at 60 minutes. A mathematical model was applied to obtain the mass transfer coefficient, diffusivity coefficient, and Henry’s constant contributing to the mass transfer rate. A GC-MS analysis was conducted to show the type of fatty acids and the fatty acid methyl ester produced by the extraction that showed the trans-esterification reaction. The lowest yield was obtained by 3:1 isopropyl alcohol to n-hexane ratio which had the lowest kca and De (4.7999×10-5 min-1 and 2.0000×10-9 cm2/min, respectively).
Algal oil; Fatty acid; Mass transfer; Spirulina sp; Ultrasound-assisted extraction
Microalgae, containing lipids,
has become one of the potential renewable energy resources (Setyawan et al., 2018; Chisti, 2008). They are potentially developing
in Indonesia, which has an extensive water area. Besides, they can grow in
fresh and saltwater (Daneshvar et al.,
2018; Clemens et al., 2011). The proteins, lipids, and carbohydrates contained in a microalga reach
6-52%, 7-23%, and 7-23%, respectively, depending on the strain type and growth
condition (Dewati et al., 2022; Kang et
al., 2011). Microalgae are
classified into Cyanophycae, Bacillariophyceae, Chrysophycae,
and Chlorophyceae based on their pigments. They also have high
photosynthetic efficiency, rapid growth rate, and small cultivation area (Clarens et al., 2010). Spirulina sp. is one of the green algae
found in various environments, like brackish water, freshwater, and seawater,
and thus easy to cultivate in Indonesia (Rilisa and Suantika, 2021)
Microalgae are the third generation of biomass
resources (Sardi et al., 2022), the
first and second of which have disadvantages. The first comes from food, such
as palm oil, corn oil, canola oil, and bean, and creates a conflict regarding
the fulfillment of food and energy (Haque et al.,
2015; Mohr and Raman, 2013). Meanwhile, the second comes
from non-food materials such as jatropha and waste biomass containing
cellulose, which require a high operational cost to produce
energy (Jamilatun et al., 2020; Balan,
2014).
Lipids in microalgae
comprise the storage (non-polar) lipids and the structural (polar) ones that
include oils (Sharma,
Schuhmann, and Schenk, 2012). The storage ones have the primary form of triglycerides as the energy source (D’Alessandro and Antoniosi-Filho, 2016). A polar lipid is the membrane’s primary structure that plays a role in metabolism (Casal and
Oliveira, 2007). Non-polar lipids, such as free
fatty acids (saturated, unsaturated, and polyunsaturated) and acylglycerol (mono-acyl,
di-acyl, and tri-acyl), can be easily esterified (Asikainen et
al., 2015). In contrast, polar lipids, such
as glycolipid and phospholipid, have long carbon chains and are highly
unsaturated, thus uneasily converted into biodiesel using conventional methods (Atadashi et
al., 2012).
The
ultrasound-assisted extraction method often functions to get algal oils from
microalgae cells by cavitation (Vilkhu et
al., 2008). It is
environmentally friendly since it takes less time and saves more energy than conventional
extraction methods (Sela, Budhijanto, and
Budiman, 2021; Adam et al., 2012). Ultrasound energy produces chemical and
physical effects because cavitation bubbles collapse. These effects can lead to
biodiesel production (Suganya,
Kasirajan, and Renganathan, 2014).
However, an organic
solvent is required for lipid extraction (Pradana et al., 2020; Wang et al., 2014) by first examining its polarity,
surface tension, viscosity, and vapor pressure (Lavilla and Bendico, 2017). Extracting polar and non-polar lipids needs a mixture of polar and
non-polar solvents like chloroform and methanol as the most frequently used
solvents for lipid extraction from any living tissue for their high speed and
nature is quantitative (Halim, Danquah, and Webley, 2012).
The oil extracted can be processed as biodiesel as a renewable energy
resource. The cetane number of biodiesel is higher than that of petroleum
diesel (Guo et al., 2016). Besides, the combustion
emission profile in biodiesel is better, meaning that it is good for the
environment. These reasons make biodiesel an excellent alternative to fossil
fuels (da-Silva et al., 2012).
Research on the
ultrasound-assisted extraction of lipids from microalgae found that the
sonication amplitude and duration of lipid extraction affected the percentage
of lipid extraction from Dunaliella sp., which had a positive
correlation (Shahi et al., 2021). The analysis of the fatty acid
profile of algal oil through an ultrasound-assisted Soxhlet extraction from
microalgae showed the potency for producing biodiesel (Wong and Shahirah, 2019). The power, time, and pulse were the most dominant factors in the yielded
oil developed by STATISTICA software (Wiyarno,
Mohd-Yunus, and Mel, 2014). However, the mass transfer phenomena during ultrasound-assisted
extraction have not been studied, especially in algal oil extraction. This
research aimed to observe the effect of the ratio of the polar solvent to the
non-polar one on the yield and the mass transfer phenomena.
2.1. Materials
The microalga used in
this study was Spirulina sp. obtained from Nogotirto Algae Park Sleman,
Yogyakarta, Indonesia. The components of this biomass were identified by the
proximate analysis, as shown in Table 1. Before the extraction, the microalga
was sifted to 0.06 mm to enlarge the contact area so that the cell walls would be
broken during the extraction process by ultrasonic waves (Liu et al., 2022; Lavilla and Bendicho, 2017).
The solvents used
were n-hexane technical grade, Merck, and isopropyl alcohol 99.5%,
Merck. The technical grade n-hexane solvent was used because it cost less than
the pro-analytic grade and is enough to extract the lipid. While isopropyl
alcohol 99.5% was used because the trans-esterification reaction was expected
to happen.
2.2. Methods
This extraction
method used an ultrasound device that contained a
generator, transducer, stirrer, and ultrasonic probe. The ultrasonic probe and
generator generated the ultrasonic waves at 100 Watt. Cavitation bubbles were
released using a transducer, producing high shear stress to break cells (solid
phase). The sample was placed in an ultrasound device for 15, 30, 45, and 60
minutes at a frequency of 25 kHz and a stirring speed of 150 rpm. The solvents
used were mixed isopropyl alcohol and n-hexane with ratios of 1:3, 2:3,
1:1, 3:2, and 3:1.
After the extraction process, the separation
process was needed to separate the solid and liquid phases through vacuum
filtration. After the filtration, the oil and solvent had to be separated. The
separation process used a distillation apparatus at 800C. The oil condensed
while the solvent evaporated. The algal oil was weighed until it got a constant
weight. After the weighing, the oil sample was analyzed using GC-MS to identify
the type of fatty acid. The scheme of the extraction process is illustrated in
Figure 1.
Figure 1 Extraction Process
The oil concentration from the extraction
process was calculated using Equation (1).
Here, Cf was the concentration of
extract in a liquid phase (g/mL), m was the mass of extract (g), and V was the
volume of liquid (mL).
3.1. Oil Yield
In this study, oil
yield refers to the essential indicator to evaluate extraction efficiency. The
oil extracted from microalgae was processed into biodiesel as a renewable
energy resource. There are two groups of solvents: polar and non-polar (Saini et al., 2021). The former included methanol, ethanol, and
isopropyl alcohol, while the latter included chloroform and n-hexane. The
solvents used in this study were chosen based on polarity (high polarity was
polar solvent, and the low polarity was non-polar solvent), boiling point
temperature to reduce energy consumption during the separation process, and
toxicity to ensure the solvent was safe (González-Fernández et al., 2020). Their properties are shown in Table 1.
Although all the solvents had suitable polarity index and boiling point
temperature, which should be below the boiling point of algal oil (about 180 0C
when using the boiling point of petroleum diesel), safety must be under
consideration.
LC50
referred to toxicity. The lower number of LC50, the more toxic the
substance. LC50 of isopropyl alcohol is extremely higher than
methanol because methanol is toxic and can form high vapor concentrations at
room temperature, which can be easily absorbed through respiration during
exposure (Moon, 2017). It is essential to choose a safer solvent to
minimize the hazard in the process and environment. Moreover, methanol causes
high environmental pollution in the transesterification process based on a life
cycle assessment study (Wahyono et
al., 2022). So, isopropyl
alcohol was chosen as the polar solvent (polarity index was 3.92) and n-hexane
as the non-polar solvent (polarity index was 0.1), although LC50
does not correlate with mass transfer phenomena.
The oil yield was calculated by Equation (2).
The mass of lipids, protein, and carbohydrates in microalgae was calculated
using the proximate analysis data shown in Table 2.
Table 1 Properties of Solvent
|
Solvent |
Polarity Index |
Boiling point |
LC50 (inhalation, rat) |
|
methanol |
5.1 |
64.7 0C |
128.2 ppm, 4 hours |
|
isopropyl alcohol |
3.92 |
82 0C |
>10,000 ppm, 6 hours |
|
chloroform |
2.7 |
61 0C |
125 ppm, 4 hours |
|
n-hexane |
0.1 |
68.7 0C |
48,000 ppm, 4 hours |
Table 2 The
results of proximate analysis of Spirulina powder
|
Component |
Content (%) |
|
Water |
11.25 |
|
Ash |
6.63 |
|
Lipid |
0.81 |
|
Protein |
48.32 |
|
Carbohydrate |
32.99 |
The extraction using
a single solvent was examined in this experiment, shown in Figure 2. Isopropyl
alcohol with a polarity index of 3.92 was used as a polar solvent, while n-hexane
with a polarity index of 0.1 was taken as the non-polar one. The yield of the
polar lipid solvent was higher than that of the non-polar one because the polar
lipids contained in the microalga were more than 50 percent of the total lipids
(Arif et al., 2019; Breuer et
al., 2013). Besides, other
polar cellular components, such as protein, pigments, and carbohydrates (Irawati et al., 2020; Agustini et al.,
2015), were also present
in this extraction product. The yield at 60 minutes of extraction time reached
1.31% using isopropyl alcohol and 0.72% using n-hexane. The yield of
n-hexane solvent was not saturated at the long extraction time, indicating the
mass transfer rate using n-hexane was relatively slow.
The effect of the
mixed solvent on the oil yielded is illustrated in Figure 3, indicating that
the ratio of the polar solvent to the non-polar one affects the yield. The
lowest yield was at the ratio of 3:1, showing that the extraction process needs
a non-polar solvent more than a polar solvent. This finding was in contrast to
the extraction yield using a single solvent. The non-polar solvent, if being
too much, can lower the yield. The ratio of the solvents, therefore, should be
optimized. Based on the experiment, the highest yield was 3.01% when the extraction
ran under the ratio of 2:3.
In an extraction
using mixed solvents (in this case, isopropyl alcohol and n-hexane), both are
added simultaneously to the microalgae biomass to extract both polar and
non-polar lipids. Besides, the aqueous phase contains non-lipid components,
such as proteins and carbohydrates (Wang et al., 2021; Halim, Danquah, and Webley, 2012).
The comparison using a single solvent and a
mixed solvent is shown in Table 3. The highest yield from a single solvent was
achieved by using isopropyl alcohol at 60 minutes of extraction (1.31%), while
the lowest yield using mixed solvent at the same extraction time was 1.46%. It
was indicated that using mixed solvent gave a higher yield.
Figure 2 Algal Oil Yield by Single Solvent Extraction
Figure 3 Algal Oil Yield by Solvent Mixture Extraction
Table 3 The Comparison of Yield Using Single Solvent and
Mixed Solvent
|
Yield at 60 minutes of extraction |
Single solvent |
Mixed solvent |
|
Maximum yield |
1.31 |
3.01 |
|
Minimum yield |
0.72 |
1.46 |
The ratio of microalgae mass to solvent
volume at the 3:2 polar to non-polar solvent ratio is illustrated in Figure 4.
Three solid-to-solvent ratios were investigated namely 1:1, 2:3, and 3:10
biomass-to-solvent ratios. The highest yield was 2.18%, achieved by the 3:10
biomass-to-solvent ratio, while the lowest yield was obtained by an extraction
process using the 1:1 biomass-to-solvent ratio.
Figure 4 Algal Oil Yield in Various Biomass to Solvent Ratios
3.2. Product Composition
Algal oils produced
by various isopropyl alcohol to n-hexane ratios were analyzed based on
the % area of the peak in the GC-MS analysis results, as shown in Figure 5. The
algal oils had three groups of components: fatty acids, hydrocarbons, and
alcohol. The classification was based on the name of the compound and its
molecular formula.
Overall, the
hydrocarbon composition was relatively constant except in the 3:1 isopropyl
alcohol to n-hexane ratio. It declined from above 50% to 42.10%.
Meanwhile, the highest alcohol peak area was found in the extracted oil with
the 3:1 isopropyl alcohol to n-hexane ratio.
The highest %peak
area of fatty acid and its derivative products was found at the 3:1 isopropyl
alcohol to n-hexane ratio, while the lowest was at the 1:3 ratio. These
findings indicated that Spirulina sp. has polar lipids more than
non-polar lipids because polar lipids pull the polar substances.
Figure 5 The Composition of Algae Oil
The fatty acid compositions are shown in Table
4. In this study, the compositions of fatty acid (FA) and fatty acid methyl
ester (FAME) in algal oil were palmitic acid, linoleic acid, methyl palmitate,
and methyl linoleate.
Table 4 The Compositions of FA and FAME in Algae Oil
|
Solvent (isopropyl alcohol to n-hexane) ratio |
FA |
FAME | ||
|
Palmitic Acid |
Linoleic Acid |
Methyl Palmitate |
Methyl Linoleate | |
|
1:3 |
11.99 |
8.61 |
2.78 |
1.17 |
|
2:3 |
8.17 |
19.53 |
3.58 |
4.65 |
|
1:1 |
7.72 |
18.79 |
2.03 |
5.86 |
|
3:2 |
6.17 |
19.15 |
2.78 |
5.12 |
|
3:1 |
5.81 |
26.87 |
6.30 |
5.69 |
The FAME yielded from the fatty acid, as
illustrated in Figure 6, reached the highest amount when using the 3:1
isopropyl alcohol to n-hexane ratio, with the highest amount of alcohol
solvent. This phenomenon indicated that the trans-esterification reaction that
reacted isopropyl alcohol and fatty acid (FA) into fatty acid methyl ester
(FAME) occurred in this extraction process.
Figure 6 FAME Yield to FA in Algal Oil
3.3. Mass Transfer Modeling
Algal oil extraction is a mass transfer
phenomenon from the solid (microalga cells) to the liquid (solvent). Such a
phenomenon can be modeled by Equation 3, and the equilibrium equation can be
approached by Henry’s law as in Equation 4 as follows:
In the solid phase, the mass
transfer can be arranged from Equation 5 to Equation 8 with the assumption that
microalga’s shape was round and the operation condition was isothermal.
Equations 3, 4, and 8 are
simultaneous differential equations that have boundary conditions, as in Equations
9, 10, and 11.
Here, kca
is volumetric mass transfer coefficient (min-1), Cf is
the concentration of lipid in a liquid phase (g/ml), Cf* is the
saturated concentration of lipid in a liquid phase (g/ml), CA is
lipid concentration in the solid phase (g/ml), H is Henry constant, r is the
radius of microalgae particles (cm), De is effective diffusivity (cm2/min).
MATLAB processed these simultaneous differential equations to get kca, De, and H constants, as shown in Table 5.
Table 5 Constants of kca, De, and H
in various solvent ratios
|
Solvent (isopropyl alcohol to n-hexane) ratio |
kca (min-1) |
De (cm2/min) |
H |
|
1:3 |
1.31×10-3 |
1.11×10-7 |
8.57 |
|
2:3 |
1.48×10-4 |
9.39×10-7 |
12.61 |
|
1:1 |
1.64×10-4 |
5.61×10-6 |
8.83 |
|
3:2 |
1.52×10-4 |
6.10×10-7 |
8.87 |
|
3:1 |
4.80×10-5 |
2.00×10-9 |
10.04 |
The highest mass transfer coefficient, Henry’s
constant, and effective diffusivity were reached when the algal oil was
extracted at the 1:3, 2:3, and 1:1 isopropyl alcohol to n-hexane ratios,
respectively. All the data obtained concluded that De and H, besides
kca, are factors in the oil yield. The lowest oil yield was obtained
by 3:1 isopropyl alcohol to n-hexane solvent ratio, which had the lowest number
of kca (4.80×10-5 min-1) and De
(2.00×10-9), while Henry's constant was the second highest (10.04).
At this solvent ratio, the GC-MS' peak area of the fatty acid result was the
highest. It indicates that other solvent ratios extracted more other compounds
than lipids.
The yield of mixed
solvent was higher than single solvent. When using a single solvent, polar
solvent achieved a higher yield than non-polar solvent. The ratio of solvents
in algal oil extraction affects the oil and substances yielded. In this study,
the highest oil yield, namely 3.01%, was obtained at the 2:3 isopropyl alcohol
to n-hexane ratio. The products yielded were not only affected by the mass
transfer coefficient but also by the effective diffusivity coefficient and
Henry's constant. Based on the extraction process yields analysis, the large
amount of the polar solvent indicated that polar lipids dominated the fatty
acid in the algal oil and were converted to biodiesel under a
trans-esterification reaction through the ultrasound-assisted extraction
process. This statement was confirmed by the high amount of fatty acid methyl
ester yielded using the highest volume of alcohol.
Adam, F., Abert-Vian, M., Peltier, G., Chemat, F., 2012. ‘Solvent-Free’
Ultrasound-Assisted extraction of Lipids from Fresh Microalgae Cells: A Green,
Clean and Scalable Process. Bioresource Technology, Volume 114, pp.
457–465
Agustini, T.W., Suzery, M., Sutrisnanto, D., Ma’ruf, W.F.,
Hadiyanto, 2015. Comparative Study of Bioactive Substances Extracted from Fresh
and Dried Spirulina sp. Procedia Environmental Sciences, Volume 23, pp.
282–289
Arif, M., Bai, Y., Usman, M., Jalalah, M., Harraz, F.A., Al-Assiri, M.S.,
Li, X., Salama, E.-S., Zhang, C., 2020. Highest Accumulated Microalgal Lipids (Polar and Non-polar) for
Biodiesel Production with Advanced Wastewater treatment: Role of Lipidomics. Bioresource
Technology, Volume 298, p. 122299
Asikainen, M., Munter, T., Linnekoski, J., 2015. Conversion
of polar and non-polar algae oil lipids to fatty acid methyl esters with solid
acid catalysts – A model compound study. Bioresource Technology, Volume 191,
pp. 300–305
Atadashi, I.M., Aroua, M.K., Abdul Aziz, A.R., Sulaiman, N.M.N., 2012.
Production of Biodiesel Using High Free Fatty Acid Feedstocks. Renewable and
Sustainable Energy Reviews, Volume 16(5), pp. 3275–3285
Balan, V., 2014. Current Challenges in Commercially
Producing Biofuels from Lignocellulosic Biomass. ISRN Biotechnology,
Volume 2014, pp. 1–31
Breuer,
G., Evers, W.A.C., de Vree, J.H., Kleinegris, D.M.M., Martens, D.E., Wijffels,
R.H., Lamers, P.P., 2013. Analysis of Fatty Acid Content and Composition in
Microalgae. Journal of Visualized Experiments, Volume 80
Casal, S., Oliveira, B., 2007. Fatty Acids Analysis by Gas
Chromatography (GC). Encyclopedia of Chromatography, Second Edition, J.
Cazes, Taylor & Francis, pp. 1-15
Chisti, Y., 2008. Biodiesel from Microalgae Beats Bioethanol. Trends
in Biotechnology, Volume 26(3), pp. 126–131
Clarens, A.F., Resurreccion, E.P., White, M.A., Colosi, L.M., 2010.
Environmental Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environmental
Science & Technology, Volume 44(5), pp. 1813–1819
Clemens, S., Aarts, M.G.M., Thomine, S., Verbruggen, N., 2013. Plant
Science: The Key to Preventing Slow Cadmium Poisoning. Trends in Plant
Science, Volume 18(2), pp. 92–99
D’Alessandro, E.B., Antoniosi-Filho, N.R., 2016. Concepts and Studies on
Lipid and Pigments of Microalgae: A Review. Renewable and Sustainable Energy
Reviews, Volume 58, pp. 832–841
da-Silva, C., Vieitez, I., Jachmanin, I., De, F., Cardozo, L.,
de-Oliveir, J.V., 2012. Non-Catalytic Production of Ethyl Esters Using
Supercritical Ethanol in Continuous Mode. Biodiesel - Feedstocks,
Production, and Applications, pp. 251–280
Daneshvar, E., Zarrinmehr, M.J., Hashtjin, A.M., Farhadian,
O., Bhatnagar, A., 2018. Versatile Applications of Freshwater and Marine Water
Microalgae in Dairy Wastewater Treatment, Lipid Extraction and Tetracycline
Biosorption. Bioresource Technology, Volume 268, pp. 523–530
Dewati, P.R., Rochmadi, Rohman, A., Budiman, A., 2022. Degradation Rate
of Astaxanthin from Haematococcus pluvialis. Food Research, Volume
6(4), pp. 254–258
González-Fernández, M.J., Manzano-Agugliaro, F.,
Zapata-Sierra, A., Belarbi, E.H., Guil-Guerrero, J.L., 2020. Green Argan Oil
Extraction from Roasted and Unroasted Seeds by Using Various Polarity Solvents
Allowed by The EU Legislation. Journal of Cleaner Production, Volume
276, p. 123081
Guo, S., Yang, Z., Gao, Y., 2016. Effect of Adding Biodiesel
to Diesel on the Physical and Chemical Properties and Engine Performance of
Fuel Blends. Journal of Biobased Materials and Bioenergy, Volume 10(1), pp.
34–43
Halim, R., Danquah, M.K., Webley, P.A., 2012. Extraction of Oil from
Microalgae for Biodiesel Production: A Review. Biotechnology Advances,
Volume 30(3), pp. 709–732
Irawati,
D., Abdillah, A.A., Pramono, H., Sulmartiwi, L., 2020. The Effect of Using
Different Polar Solvents on the Stability of Thermal Extraction Phycocyanin
From Spirulina Platensis. IOP Conference Series: Earth and Environmental
Science, Volume 441(1), p. 012050
Haque,
S.M., Bhat, A.H., Khan, I., 2015. Biomass: An Ageless Raw Material for
Biofuels. Agricultural Biomass Based Potential Materials. Springer
International Publishing, pp. 435–454
Jamilatun, S., Budhijanto, Rochmadi, Yuliestyan, A.,
Aziz, M., Hayashi, J., Budiman, A., 2020. Catalytic Pyrolysis of Spirulina Platensis
Residue (SPR): Thermochemical Behavior and Kinetics. International Journal
of Technology, Volume 11(3), pp. 522–531
Kang, K.H., Ryu, B.M., Kim, S.K., Qian, Z.J., 2011. Characterization of
Growth and Protein Contents from Microalgae Navicula incerta with the
Investigation of Antioxidant Activity of Enzymatic Hydrolysates. Food
Science and Biotechnology, Volume 20(1), pp. 183–191
Lavilla, I., Bendicho, C., 2017. Fundamentals of Ultrasound-Assisted
Extraction. Water Extraction of Bioactive Compounds: From Plants to Drug
Development, Elsevier, pp. 291–316
Liu, Y., Liu, X., Cui, Y., Yuan, W., 2022. Ultrasound for Microalgal Cell Disruption and Product Extraction:
A review. Ultrasonics Sonochemistry,Volume 87, p. 106054
Mohr, A., Raman, S., 2013. Lessons from First Generation Biofuels and
Implications for the Sustainability Appraisal of Second Generation Biofuels. Energy
Policy, Volume 63, pp. 114–122
Moon, C.S., 2017. Estimations of the Lethal and
Exposure Doses for Representative Methanol Symptoms in Humans. Annals of
Occupational and Environmental Medicine, Volume 29(1), pp. 1–6
Pradana, Y.S., Dewi, R.N., Livia, K.D., Arisa, F.,
Rochmadi, Cahyono, R.B., Budiman, A., 2020. Advancing Biodiesel Production from
Microalgae Spirulina sp. by a Simultaneous Extraction–transesterification
Process using Palm Oil as a Co-solvent of Methanol. Open Chemistry, Volume
18(1), pp. 833–842
Rilisa, C., Suantika, G., 2021. Biological, Technical,
and Financial Feasibilities Study of Spirulina sp. Biomass Production with
Modified Commercial Medium in Indonesia. 3BIO: Journal of Biological
Science, Technology and Management, Volume 3(2), pp. 12–21
Saini,
R.K., Prasad, P., Shang, X., Keum, Y.-S., 2021. Advances in Lipid Extraction
Methods—A Review. International Journal of Molecular Sciences, Volume
22(24), p. 13643
Sardi, B., Ningrum, R.F., Ardiansyah, V.A., Qadariyah,
L., Mahfud, M., 2022. Production of Liquid Biofuels from Microalgae Chlorella
sp. via Catalytic Slow Pyrolysis. International Journal of Technology,
Volume 13(1), pp. 147–156
Sela, K., Budhijanto, W.,
Budiman, A,, 2021. Protein Extraction from Spirulina platensis by Using
Ultrasound Assisted Extraction: Effect of Solvent Types and Extraction Time. Key
Engineering Materials, Volume 872, pp. 33–37
Setyawan, M., Budiman, A., Mulyono, P., Sutijan, 2018.
Optimum Extraction of Algae-oil from Microalgae using Hydrodynamic Cavitation. International
Journal of Renewable Energy Research, Volume 8(1), pp. 451–458
Shahi, T., Zenouzi, A., Beheshti, B., Almasi, M., 2020. Comparison of
Four Lipid Extraction Methods from Microalgae Dunaliella sp. for Biodiesel
Production. Iranian Journal of Chemistry and Chemical Engineering,
Volume 39(4), pp. 371–378
Sharma, K.K., Schuhmann, H., Schenk, P.M., 2012. High Lipid
Induction in Microalgae for Biodiesel Production. Energies, Volume 5(5),
pp. 1532–1553
Suganya, T., Kasirajan, R., Renganathan, S.,
2014. Ultrasound-enhanced Rapid in Situ Transesterification of Marine
Macroalgae Enteromorpha Compressa for Biodiesel Production. Bioresource
Technology, Volume 156, pp. 283–290
Vilkhu, K., Mawson, R., Simons, L., Bates, D., 2008. Applications and
Opportunities for Ultrasound Assisted Extraction in the Food Industry - A
Review. Innovative Food Science and Emerging Technologies, Volume 9(2),
pp. 161–169
Wahyono, Y., Hadiyanto, Budihardjo, M.A., Hariyono, Y.,
Baihaqi, R.A., 2022. Multifeedstock Biodiesel Production from a Blend of Five
Oils through Transesterification with Variation of Moles Ratio of Oil:
Methanol. International Journal of Technology, Volume 13(3), pp. 606–618
Wang,
J., Yu, Z., Yin, F., Li, D., Liu, H., Song, L., Zhou, D., 2021. Comparison of Different
Solvents for Extraction of Oils from By?Products of Shrimps Penaeus Vannamei
and Procambarus Clarkia. Journal of Food Processing and Preservation,
Volume 45(9), pp. 1-9
Wang, M., Yuan, W., Jiang, X., Jing, Y., Wang, Z., 2014. Disruption of
Microalgal Cells Using High-frequency Focused Ultrasound. Bioresource
Technology, Volume 153, pp. 315–321
Wiyarno, B., Mohd-Yunus, R., Mel, M., 2014. A Study on The Ultrasonic
Oil Extraction and Insitu Transesterification of Microalgae Biodiesel. CHEMICA:
Jurnal Teknik Kimia, Volume 1(2), p. 41
Wong, Y.C., Shahirah, R., 2019. Effect of Different Solvent and Ratio
Towards Microalgae Oil Production by Ultrasonic Assisted Soxhlet Extraction
Techniques. Oriental Journal of Chemistry, Volume 35(4), pp. 1377–1383
