Composting of Oil Palm Empty Fruit Bunches by Microbial Inoculant
Corresponding email: zaidazahari@ums.edu.my
Published at : 28 Jul 2023
Volume : IJtech
Vol 14, No 5 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i5.5907
Zahari, N.Z., Tuah, P.M., Zulkifli, N.H.C., Cleophas, F.N., 2023. Composting of Oil Palm Empty Fruit Bunches by Microbial Inoculant. International Journal of Technology. Volume 14(5), pp. 1081-1092
| Nur Zaida Zahari | Faculty of Science and Natural Resources Universiti Malaysia Sabah, UMS Road, 88400 Kota Kinabalu, Malaysia |
| Piakong Mohd Tuah | Bioreg Technology Solutions Sdn. Bhd., Block H, Lot 3, 2nd Floor, Kian Yap Road, Inanam Capital, 88450 Kota Kinabalu, Malaysia |
| Nur Hazwani Che Zulkifli | Faculty of Science and Natural Resources Universiti Malaysia Sabah, UMS Road, 88400 Kota Kinabalu, Malaysia |
| Fera Nony Cleophas | Faculty of Science and Natural Resources Universiti Malaysia Sabah, UMS Road, 88400 Kota Kinabalu, Malaysia |
Composting
is a natural process that occurs as a result of microbial succession and marks
the degradation and stabilization of organic matter in waste. The use of
microbial additives during composting is believed to be highly efficient, as it
increases the production of various enzymes, resulting in a faster rate of
waste degradation. The process converts waste into a humus-like substance that
can enhance the physical, chemical, and biological properties of soil. In this
study, to assess the contribution of microorganisms to the composting process,
we selected the thermophilic bacteria degrading Bacillus subtilis-ReK1Hs-Cr1 as the inoculant. The analysis of
temperature, pH, moisture, microbial population, compost maturity, seed
germination test, and C:N ratio were all monitored during the composting
process. Physical changes in the compost, such as color, texture, and smell,
were also observed. The findings showed that the inoculation of this
thermophile with the addition of chicken manure was able to raise the
temperature up to 67.3oC, which may have resulted in the elimination
of pathogens, which is above the recommended temperature level of 55oC
for 3 consecutive days. There was an increase in the microbial population (4.6
x105 -2.9 x107) at the start of the experimental works
and the temperature decreased as the composting process proceeded because of
the maturity phase. The pH; 7–9 and moisture content; 51–63 % of compost
materials were in the range of optimum composting conditions. The weight of the compost
materials in all three treatments, except for the control, decreased by 47% to
55%. The color of the compost turned to dark brown, the obnoxious odor was
eliminated, and the texture changed to lose and easy pulverize at the final
stage of composting. High germination index (GI) was obtained from the test,
indicating that the final product is free from phytotoxic substances and thus
suitable as a soil conditioner/fertilizer. Therefore, B. subtilis ReK1Hs-Cr1 is the best candidate
as an inoculant added with chicken manure to degrade OPEFB on a large scale
using windrow technology.
Bacillus subtilis-ReK1Hs-Cr1; Composting; Germination index; Oil palm empty fruit bunch; Thermophilic strain
Oil palm production is a major agricultural industry in Malaysia. As a result, there has been an increase in the amount of waste generated by the oil palm industry, especially empty fruit bunches (EFB). Solid wastes from oil palm plantations, such as trunks, fronds, and oil palm empty fruit bunches (OEEFB), are a source of concern for the environment. As a result, composting is preferred and environmentally sound, whereby organic waste is reduced to organic fertilizer and soil conditioners through biological processes (Shukor et al., 2018; Pan, Dam, and Sen, 2012). In composting, the higher-plant material breaks down under the influence of aerobic thermophilic microorganisms present in the waste to a material rich in organic nutrients. Oil Palm Empty Fruit Bunch (OPEFB) fiber is a lignocellulosic waste from palm oil mills that contains 12% lignin and 75% cellulose (Hermansyah et al., 2019). Lignocellulose components are the main building block of plant cell walls where the cellulose fibrils are embedded in an amorphous matrix of lignin and hemicellulose (Zoghlami and Paes, 2019). Lignin is a structural polymer found in vascular plants, which plays essential functions in their survival. It provides rigidity to the plant and binds plant cells together, imparting resistance to impact, bending, and compression. However, cellulose is a highly regular polymer of any hydro-D-glucopyranase unit linked with ß (1-4) linkage, and this molecule can be easily metabolized by various cellulolytic microbes. Bacteria play a significant role in the humification processes associated with soil and compost. The enzymatic ability to cleave alkyl-aryl ether bonds enables bacteria to degrade oligomeric and monomeric aromatic compounds released during lignocellulose degradation. Composting has been used as a system for the disposal of organic waste, to produce organic fertilizer, and to improve soil biological fertility. Zulkepli and Tuah (2008) reported thermophilic bacteria had the potential to enhance the biocomposting of OPEFB which can reduce the weight of OPEFB and cause lignocellulose loss in 7 days of degradation. He also reported that the maximum temperature achieved during the composting of OPEFB by lignocellulose-degrading bacteria was only 45oC which did not meet the minimum requirement of 55oC for three consecutive days sanitized compost material as suggested by Jones and Martin (2003). The four most commonly used composting methods in the industry are open static piles, turning windrows and piles, aerated static piles, and in-vessel systems. Among these options, an in-vessel composting system has many advantages because it requires less space and provides better control over agitation, aeration, and mixing of the compost materials. Typically, two phases are engaged in in-vessel composting systems: a high-rate phase in the vessel composter and a curing phase in an outside composting pile. In this study, a locally isolated thermophilic lignocellulose-degrading bacteria from a hot spring (Bacillus subtilis ReK1Hs-Cr1) (Zulkepli and Tuah, 2008) were investigated for its capability to enhance composting of shredded OPEFB by using an in-vessel composting system. Non-inoculant and inoculant bin-type composting of OPEFB were compared by monitoring parameters which include temperature, microbial population, pH, C: N ratio, and Germination Index during the 90-day period.
2.1. Organism
maintenance and preparation
Bacteria used in this work was the lignocellulose-degrading thermophilic
strain Bacillus
subtilis-ReK1Hs-Cr1 obtained from a hot spring in Ranau, Sabah (Zulkepli and Tuah, 2008). This strain has
been selected due to its capability to sustain high temperatures, thus helping
in elevating the temperature of compost materials and ensuring the elimination
of both animal and plant pathogens during the composting process. The
organism was maintained on Nutrient Agar (2 g/L yeast extract, 2 g/L meat
extract, 5 g/L peptone, 4 g/L NaCl, 10 g/L glucose, and 12 g/L agar in a liter)
at 4°C (Zahari et al., 2021a).
Subcultures were made weekly and cultivated at 40°C. To achieve successful and
rapid composting of shredded OPEFB, inoculation of bacteria was needed. The
sterilized raw materials were inoculated with 10% broth (Zahari et al., 2022) inoculum (107 CFU/mL)
of Bacillus subtilis-ReK1Hs-Cr1.
2.2. Composting
process
Lab-scale in-vessel composting was carried out in accordance with Figure
1. Black bins with a capacity of 69 L and a height of 0.65 m (65 cm) were
prepared. Approximately 10 holes with diameters of 1.5 to 2.0 cm were drilled
at the bottom of each bin to collect leachate and similar holes were drilled on
the sides of the bins for aeration purposes. The composting process involved
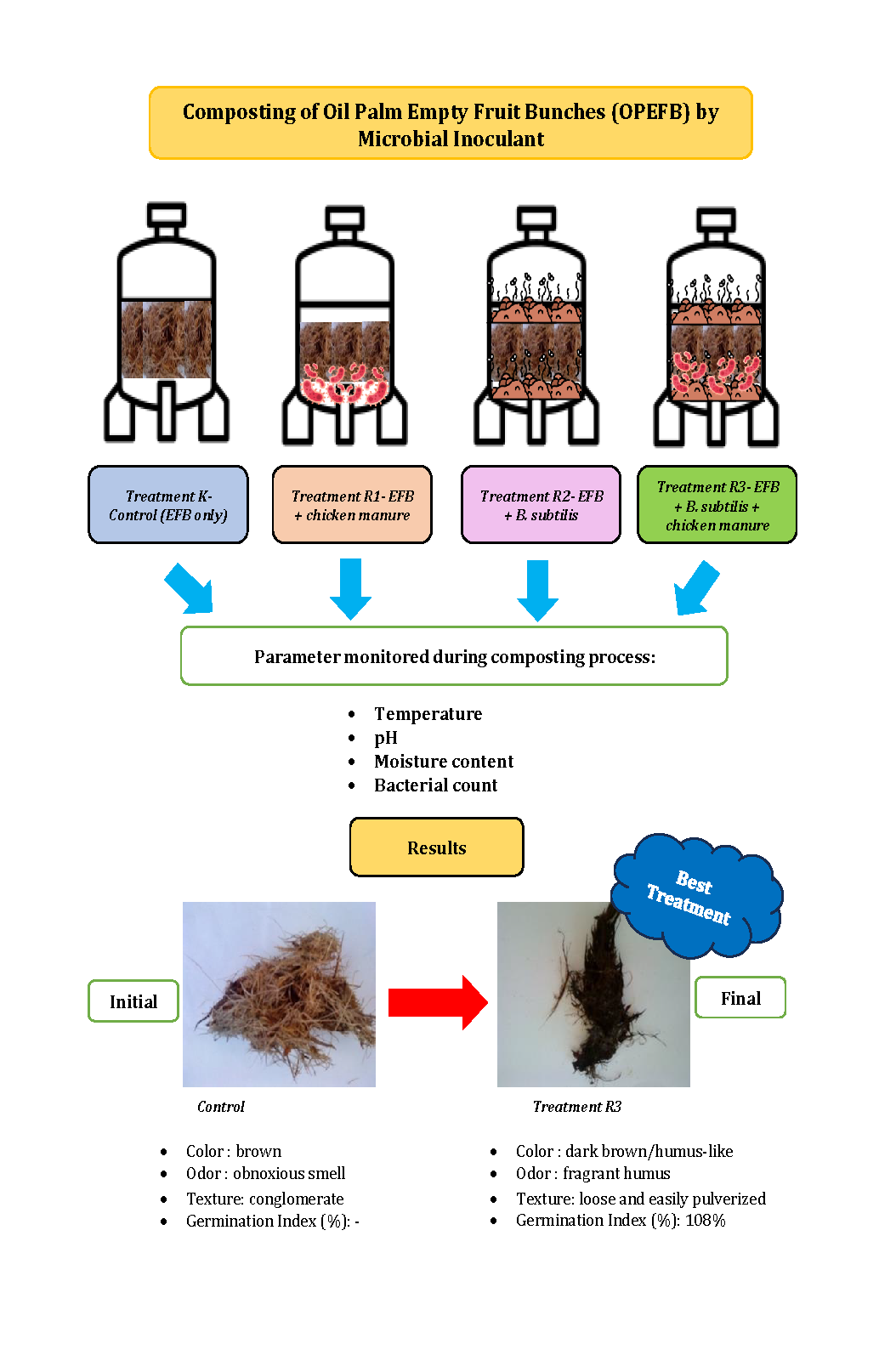
four treatments, each with two duplicates, as shown in Table 1.
Table 1 Various treatments
carried out in this study.
Figure 1 In-vessel composting (a)
(bin-type) and (b) arrangement of EFB and chicken manure in the bin.
2.3. Sampling and analysis
The reduction weight of the compost along with color
and texture, was observed at the initial and the end of the composting process.
The temperature of the compost was recorded daily at the surface, middle, and
bottom of the layer by using a digital temperature probe. pH value was analyzed
by using a pH meter (1: 10 waters: extract). Moisture content was analyzed by
drying 10 g of the sample at 1200C until a constant weight was
obtained. In
maintaining the moisture content of all treatments, inoculants were added
according to the weight of the compost materials, which is 5 mL for every
kilogram of waste in the compost weekly. For biomass concentration, the
microbial population (CFU/mL) was tested every 15 days. The Spread Plate
Technique was used to obtain the CFU/mL after 24 h at 40°C (Taliesin and John, 2021). The inoculant was added on
a weekly basis. Leachate was collected and added back into the compost material
to control the moisture content. The
carbon and nitrogen contents were analyzed using CNHS/O analyzer 2000 and
Inductively Coupled Plasma (ICPOES, Perkin Elmer, USA). All samples were done in
triplicates to obtain accurate data. The seed germination index (GI) was
carried out to determine compost maturity and stability. 10 g of green bean
seed ore were placed on filter paper soaked in 5 mL of compost extract. The
petri dish was placed in a dark room at room temperature. For control, 1 g of
seed was soaked in distilled water. The experiment was conducted in triplicates
and incubated for 3 days. After incubation, the root length and seed
germination index (GI) was calculated using the equation by Fetouh and Hassan (2014) below:
3.1. Physiochemical changes during the
co-composting process
The changes in temperature during the
90-day composting process of different treatments are shown in Figure 2 (a).
There are four stages included in this process which are mesophilic,
thermophilic, cooling, and maturity compost. The duration of each stage depends
on the initial composition of the mixture in a treatment bin. In the mesophilic
phase, a variety of bacteria, actinomycetes, and fungi quickly break down the
C-rich substrates at moderate temperatures, usually between 15 and 40°C. Heat
is produced by this aerobic metabolism. As time passed, the temperature rose
rapidly to more than > 45°C (Thermophilic phase) in less than 15 hours
during the initial stages. The results show that the temperature was increased
in all treatments studied, describing a peak of microbial activity. For
Treatment 1 (R1), (R2), and (R3), the maximum temperature reached was 58.8oC,
56.8oC and 67.3°C on day four, respectively. The thermophilic phase
of Treatment 3 (R3) was maintained for 14 days, while for other treatments, the
thermophilic phase was maintained for only 8 days. While for the control (K),
the thermophilic phase only lasted 4 days, and the maximum temperature only
reached 50.2°C. This shows that the addition of an inoculant of bacterial
strain and chicken manure has a positive effect on the composting, especially
during the thermophilic phase, that help in elevating the temperature of
compost materials during composting process. The high temperature achieved
during the composting process ensured the elimination of both animal and plant
pathogens. Only three to four days at a temperature of 55°C was sufficient for
eliminating the pathogens (Zhao, Ta, and Wang, 2017). Kim et al. (2019) reported
that pathogens can be killed at 55°C. Additionally, almost all pathogens are
eliminated in a few days making the compost product suitable for agricultural
use. Besides, a rise in temperature during composting are the consequence of
the breaking down of the readily available organic matter and nitrogen
compounds by microorganism (Raut et al., 2008). As time
increase, the organic matter becomes more stabilized, thus slower the microbial
activity and decomposition rate. A steady temperature was achieved at the last
stages of composting, indicating that less degradation (cooling phase) process
took place. The final maturing phase is characterized by lower temperatures
below 25°C and reduced oxygen uptake rates of aerobic microorganisms. During this
stage, degradation of the more refractory organic compounds continues, and soil
meso and macrofauna enter.
Figure 2(b) illustrates the weekly pH
changes of the composting process for the different treatments over the 90-day
period. The initial pH values of all treatments of the composting process were
nearly neutral, which were 7.6 for (R1), 7.5 for (R2), 7.5 for (R3), and 7.7
for Control (K). During the composting process, the pH values for all
treatments increased until week 3. For Treatment 1, the pH of the compost
material gradually increased to 8.7 at week three from the initial pH of 7.5.
The pH value at week three was observed to be the highest pH value, 8.7,
throughout the composting process. Then, the pH value slightly decreased from
week four to week six. This trend has been observed in all treatments. The pH
rise was due to the organic acid volatilization and microbial decomposition
(mineralization) that release ammonia from the organic nitrogen sources (Umsakul, Dissara, and Srimuang, 2010). pH for (R1)
has been decreased to 7.1 at week 11 then the value raised again to 8.8 in the
final stage of the composting process. A similar trend was observed in (R2),
(R3), and Control (K). At the final stage of the composting process, the
results obtained for (R1), (R2), and (R3) and Control (K) were 8.8, 8.1, 8.5, and 8.1
respectively.
Figure 2 Profile of physiochemical elements that use different treatment conditions. (a) Temperature changes during composting. (b) pH changes during different waste substrates. (c) Changes in moisture content during composting
Previous
research found that microbial activity increased the likelihood of achieving a
suitable pH range of 5.5–9.0, while the composting process is most effective at
pH values between 6.5 and 8.0 (Pan, Dam, and Sen, 2012). The pH of all treatments was alkaline
and stable due to ammonia generation, which could improve the composting
process by inhibiting pathogenic fungi that prefer acidic conditions (Saidi et al.,
2008).
Figure 2 (c) shows the weekly moisture content of
the composting process of different treatments during the 90-day period.
Moisture appears to be a significant component controlling microbial activity (Anastasi, Varese, and Marchisio, 2005), as low moisture content hinders the growth of
helpful microbes (Tiquia, 2010), whereas excessive moisture might cause anaerobic conditions, resulting
in the creation of unpleasant odors and poisonous volatile compounds (Saidi et al., 2008). The
moisture content of Treatment 1 (R1), Treatment 2 (R2), Treatment 3 (R3), and
Control (K) was shown as 51 %, 56 %, 52 %, and 55 %, respectively. The moisture
content for all treatments was increased when the temperature of the compost
was decreased. This high moisture level in all treatments resulted in a cooling
effect. This is due to the gaseous exchange by reducing diffusion and
consequently constraining oxygen uptake by microbes, thus resulting in lower
microbial activity (Zahari et al., 2021b). The average moisture content for Treatment 1 (R1)
was 59.5 % throughout the composting process. The moisture content was
maintained in the range of 52 % to 62 % for the Control (K) throughout the
composting process. The moisture content for Control (K) decreased from 62 % at
the begining to 55% during the final stage of the composting process. For (R2),
the moisture content obtained in the initial stage was 58 %. The moisture
content slightly increased to 58 % at week 1. The moisture content showed a
gradual decrease at week two to 44 % even though inoculants were added to all
bins as usual and increased again to the optimal range (61%). At week three,
there was a slight increase and decrease in moisture content of (R2) until week
11. At week 11, the moisture content noted an obvious decrease to 48 % and
continued to decrease to 47 % in week 12. The decrease in moisture content may
be caused by the decrease in microbial activity in compost, which has a direct
effect on a high content of organic matter (Kazami et al.,
2016). Generally, the moisture
content for all treatments showed a decreasing trend till the end of the
composting process. The addition of inoculants into the compost materials is
adequate to maintain the moisture content of the treatments. Occasionally,
leachate from the compost was collected and added to the compost materials to
maintain the moisture content. A comparative study in Table 2 showed the
performance of various compost processes using different microbial inoculants
within the present work.
Table 2 Summary of the previous
studies by different microbial inoculants used for composting of EFB
3.2. Bacterial count
The microbial community of different treatments was
studied during the composting process. Bacteria and fungi that constitute the
microbial community structure of the composting environment are present and
active during the composting process. Different bacteria and fungi can have a
beneficial or detrimental impact on the entire composting process. Their
diversity also hints at the composting mechanisms at work. From Figure 3, which
shows the microbial population of different treatments during the 90-day composting
process, the microbial population in the compost material of all treatments was
almost the same for (R1), (R2), (R3), and Control (K), which are 7.2×106 – 2.8×107,
5.0×105 – 2.9×107, 4.6×105 – 2.9×107 and 1.5×106 – 2.9×107 CFUmL-1, respectively. This is because the
compost materials from all treatments were from the same source. The population
of microorganisms is slightly decreased for all treatments from day 1 until day
15 because the high temperature during the thermophilic phase of composting
(> 45°C) killed the pathogens as well as other microorganisms in the
compost. The microbial population, therefore, continued decreasing until the
end of the composting process. This is because the microbial cannot survive
without nutrients and oxygen (Riedel et
al., 2013). While for
the control, the microbial population decreased in week 45, and the compost
materials were in the mesophilic phase. In this phase, it is most suitable for
most of the microorganisms to grow and reproduce. Thus, the population of
microorganisms in the compost increased again.
Figure 3 Profile of microbial population according to the
respective composting treatment.
3.3.
Compost stability and maturity
Figure 4 Germination Index of all treatments of shredded
OPEFB composting during the 90-day period.
3.4.
Weight reduction of the compost material
The initial weight of the compost materials in all
treatments during the composting process was 76.7 kg, 60.3 kg, 76.6 kg, and
59.8 kg for (R1), (R2), and (R3) and Control, respectively. The weight of the compost material in Treatment 3
showed a significant decrease in weight to 33.3 kg, which is a 57 % reduction
compared to the initial weight. The weight of the compost materials of (R1),
(R2), and Control (K) has been reduced by 54 %, 55%, and 34 %, respectively.
The weight reduction of the compost materials in all treatments was associated
with the evaporation and leaching of moisture (Jain, Daga, and Kalamdhah, 2019).
Figure 5 The
condition of compost materials at the beginning and final stage of the
composting process. (a)
OPEFB before composting, (b) Control (K) after composting; (c) Treatment 1 (R1)
after composting; (d) Treatment 2 (R2) after composting, and (e) Treatment 3 (R3) after composting
The sample taken to test the pH,
moisture content, colony forming units, carbon to nitrogen ratio, and as well
as tested compost maturity through seed germination tests from time to time has
also contributed to the reduction in weight of compost materials in all
treatments. Figure 5 (a)- (e) shows the condition of compost materials at the
beginning and final stages of the composting process. During the composting
process, gradual changes in the texture and other characteristics of the
shredded OPEFB were observed after 90 days. The changes in color, odor, and
texture for (R1), (R2), (R3), and Control (K) were the same. The color of the
compost materials changed from filemot at the beginning to dark brown
humus-like in the final stage of the composting process. This result is
comparable to a study by Pan, Dam, and Sen (2012), which reported a black-colored
humus-like substance developed after 120 days of decomposition of common
organic wastes inoculated with three bacteria, Bacillus subtilis B1U/1, B.
subtilis D3L/1, and Pseudomonas sp. RAT/5. The compost material
produced an obnoxious smell at the initial stage. As the composting process
proceeds, the compost becomes fragrant humus at the final stage of the
experiment. Lastly, the texture of the compost materials changes from conglomerate
become loose and easily pulverized at the end of the composting process.
Composting is the most
widely used technology for the biological stabilization of organic solid waste,
owing to its perceived ease of operation in comparison to anaerobic procedures.
However, the proper evolution of degrading processes must be continuously
monitored, as standard criteria for compost quality evaluation usually provide
insufficient or incorrect information, which can be damaging to process
operation and performance. The selection of the most appropriate raw material
is critical for successful composting, as it contributes to the process’s
operation and performance. This study highlights that thermophilic strains Bacillus
subtilis-ReK1Hs-Cr1 were able
to raise the temperature up to 63.7oC, which may have resulted in
the elimination of pathogens. A subsequent decrease in pile temperature at the
end of composting correlates well with other characteristics of a compost used
to evaluate its stability or maturity in a compost. The pH, 7–9, and moisture
content; 51–63 % of compost materials were in the range of optimum composting
conditions. The weight of the compost materials in all three treatments
excluding control was reduced by approximately 47–55 %. The color of the
compost was turned to dark brown; the obnoxious odor was eliminated, and the
texture changed to lose and easily pulverize at the final stage of composting.
The high germination index obtained from the test indicated that the final
product is free from phytotoxic substances and thus suitable as a soil
conditioner/fertilizer. Some suggestions can be made to improve the efficiency
of composting. The performance of composting can be further enhanced by
improving the turning/tilting process, such as by using a mechanical tilting
mechanism. Furthermore, the compost's quality as a bio-fertilizer
must be confirmed through detailed analysis of other physicochemical and
microbiological parameters, as well as studies on larger-scale processes.
Anastasi, A., Varese, G.C., Marchisio, V.F.,
2005. Isolation and Identification of Fungal Communities in Compost and
Vermicompost. Mycologia, Volume 97(1), pp 33–44
Banegas, V., Moreno, J.L., Garcia, C., Leon, G., Hernández, T., 2007. Composting
Anaerobic and Aerobic Sewage Sludges using Two Proportions of Sawdust. Waste Management Volume 27(10), pp 1317–1327
Fetouh, M.I., Hassan, F.A., 2014. Seed Germination Criteria and Seedling Characteristics of Magnolia
Grandiflora L. Trees After Cold Stratification Treatments. International
.Journal Current Microbiology & Applied Science, Volume 3(3), pp. 235–241
Gigliotti, G., Valentini, F., Erriquens, F.G.,
Said-Pullicino, D., 2005. Evaluating the Efficiency of the Composting Process.
A Comparison of Different Parameters. Geophysical Research Abstracts,
Volume 7, pp. 1–8
Hermansyah, H., Putri, D.N., Prasetyanto, A., Chairuddin, Z.B., Perdani,
M.S., Sahlan, M., Yohda, M., 2019. Delignification of Oil Palm Empty Fruit
Bunch using Peracetic Acid and Alkaline Peroxide Combined with the Ultrasound. International
Journal of Technology, Volume 10(8), pp. 1523–1532
Jain, M.S., Daga, M., Kalamdhah, A.S., 2019. Variation in The Key Indicators
During Composting of Municipal Solid Organic Wastes. Sustainable Environment
Research, Volume 29, pp. 1–8
Jones,
P., Martin, M., 2003. A Riview of The Literature on The Occurance And
Survival Of Pathogens of Animals and Human In Green Compost-Research Report.
The Waste and Resources Action Programe, Institute of Animals Health, UK
Kazemi, K., Zhang, B., Lye, L.M., Cai, Q., Cao, T.,
2016. Design of Experiment (DOE) Based. Screening of Factors Affecting
Municipal Solid Waste (MSW) Composting. Waste Management, Volume 58, pp.
107–117
Kim, C., Alrefaei, R., Bushlaibi, M., Ndegwa, E.,
Kaseloo, P., Wynn, C., 2019. Influence of Growth Temperature on Thermal
Tolerance of Leading Foodborne Pathogens. Food Science and Nutrition, Volume
7(12), pp. 4027–4036
Krishnan, Y., Bong, C., Azman, N., Zakaria, Z.,
Othman, N., Abdullah, N., Ho, C., Lee, C., Hansen, S., Hara, H., 2017. Co-composting
of Palm Empty Fruit Bunch and Palm Oil Mill Effluent: Microbial Diversity and
Potential Mitigation of Greenhouse Gas Emission. Journal of Cleaner
Production, Volume 146, pp. 94–100
Lim, L.Y., Chua, L.S., Lee, C.T., 2015. Effects
of Microbial Additive on The Physiochemical and Biological Properties of Oil
Palm Empty Fruit Bunches Compost. Journal of Engineering Science and Technology, Volume 2015, pp. 10–18
Pan, I.,
Dam, B., Sen, S.K. 2012. Composting of Common Organic Wastes Using Microbial
Inoculants. 3 Biotech, Volume 2, pp. 127–134
Raut, M.P., William, P., Bhattacharyya, J.K.,
Chakrabarti, T., Devotta, S., 2008. Microbial Dynamics and Enzyme Activities
During Rapid Composting of Municipal Solid Waste-A Compost Maturity Analysis
Perspective. Journal of Bioresource Technology, Volume 99(14), pp.
6512–6519
Riedel, T.E., Berelson, W.M., Nealson, K.H., Finkel,
S.E., 2013. Oxygen Consumption Rates of Bacteria Under Nutrient-limited
Conditions. Applied Environment Microbiology, Volume 79 (16), pp.
4921-4931
Saidi, N., Cherif, M., Jedidi, N., Fumio, M.,
Boudabous, A., Hassen, A., 2008. Evolution of Biochemical Parameters During
Composting of Various Waste Compost. American Journal of Environmental
Sciences, Volume 3(3) pp 332–341
Shukor, J.A., Omar, M.F., Kasim, M.M.,
Jamaludin, M.H., Naim, M.A., 2018. Assessment of Composting Technologies for
Organic Waste Management. International Journal of Technology, Volume 9(8),
pp. 1579–1587
Siddiquee, S. Shafawati, S., Naher, L., 2017. Effective
Composting of Empty Fruit Bunches Using Potential Trichoderma Strains. Biotechnology
Reports, Volume 13, pp 1–7
Taliesin, L., John, G., 2021. Incubation
Temperature and Culture Medium Formulation Impact the Accuracy of Pour-Plate
Technique for the Enumeration of Industrial Bacillus assemblages. Journal of
Microbiological Methods, Volume 186, pp. 1-6
Tiong, R.C., 2011. Composting of Oil Palm
Blanks with The Addition of Horse Manure And A Thermophilic Strain, Bacillus
licheniformis, NAS3dHs-Cr1. Bachelor Dissertation, Universiti Malaysia
Sabah
Tiquia, S.M., 2010. Reduction of Compost Phytotoxicity During The
Process of Decomposition. Chemosphere, Volume 79(5), pp 506–512
Umsakul, K., Dissara,
Y., Srimuang, N., 2010. Chemical Physical and Microbiological Changes
During Composting of The Water Hyacinth. Pakistan Journal of Biological
Sciences, Volume 13(20), pp. 985–992
Zahari, N.Z., Baharudzaman, E.H., Tuah, P.M.,
Cleophas, F.N., Vun, L.W., 2021b. Characterization of Polycyclic Aromatic
Hydrocarbons and Bioaugmentation Potential of Locally Isolated Beneficial
Microorganisms Consortium for Treatment of Tar-Balls. Environment and Natural Resources Journal, Volume 19(3), pp 246–254
Zahari, N.Z., Tuah, P.M., Rahim, S.A., 2021a.
Inoculation of Bacillus Cereus Enhance Phytoremediation Efficiency of Pistia
Stratiotes and Eichhornia Crassipes in Removing Heavy Metal Pb. In: IOP
Conference Series: Earth and Environmental Science, Volume 847 (1), pp. 1–9
Zahari, N.Z., Yan, G.P., Tuah, P.M., Rahim,
S.A., 2022. Effects of Single and Consortia Inoculants on the Biodegradation
Efficiency of Crude Oil in Seawater. International Journal of Technology, Volume
13(2), 2022. pp. 422–431
Zhao,
S.X., Ta, N., Wang, X.D., 2017. Effect of Temperature on the Structural and
Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock
Material. Energies, Volume 10(9), pp. 1–15
Zoghlami, A., Paës, G., 2019. Lignocellulosic
Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Frontiers in
Chemistry, Volume 12, p. 874
Zulkepli,
N.H.C., Tuah, P.M., 2008. Isolation and Characterization of lignocelluloses
Degrading Thermophilic Bacteria for Biocomposting of Oil Palm Empty Fruit
Bunch, In: Proceeding International Conference on Environmental Research
and Technology (ICERT 2008), Universiti Sains Malaysia, Penang
