Sustainable Synthesis of Copper Oxide Nanoparticles Using Aquilaria Malaccensis (Agarwood) Leaf Extract as Reducing Agent
Corresponding email: alicedcruz@yahoo.com
Published at : 19 Oct 2022
Volume : IJtech
Vol 13, No 5 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i5.5845
Valan, S.L., Cruz, A.E.D., Jacob, P.J., Djearamane, S., 2022. Sustainable Synthesis of Copper Oxide Nanoparticles Using Aquilaria Malaccensis (Agarwood) Leaf Extract as Reducing Agent. International Journal of Technology. Volume 13(5), pp. 1115-1125
| Sathia Lingam Valan | School of Applied Sciences, Faculty of Engineering, Science and Technology, Nilai University, Persiaran Universiti, Putra Nilai 1, Persiaran Kolej Bbn, 71800 Nilai, Negeri Sembilan, Malaysia |
| Alice Escalante De Cruz | School of Applied Sciences, Faculty of Engineering, Science and Technology, Nilai University, Persiaran Universiti, Putra Nilai 1, Persiaran Kolej Bbn, 71800 Nilai, Negeri Sembilan, Malaysia |
| Patricia Jayshree Jacob | School of Applied Sciences, Faculty of Engineering, Science and Technology, Nilai University, Persiaran Universiti, Putra Nilai 1, Persiaran Kolej Bbn, 71800 Nilai, Negeri Sembilan, Malaysia |
| Sinouvassane Djearamane | Department of Biomedical Science, Faculty of Science, Universiti Tunku Abdul Rahman, Kampar, 31900, Perak, Malaysia |
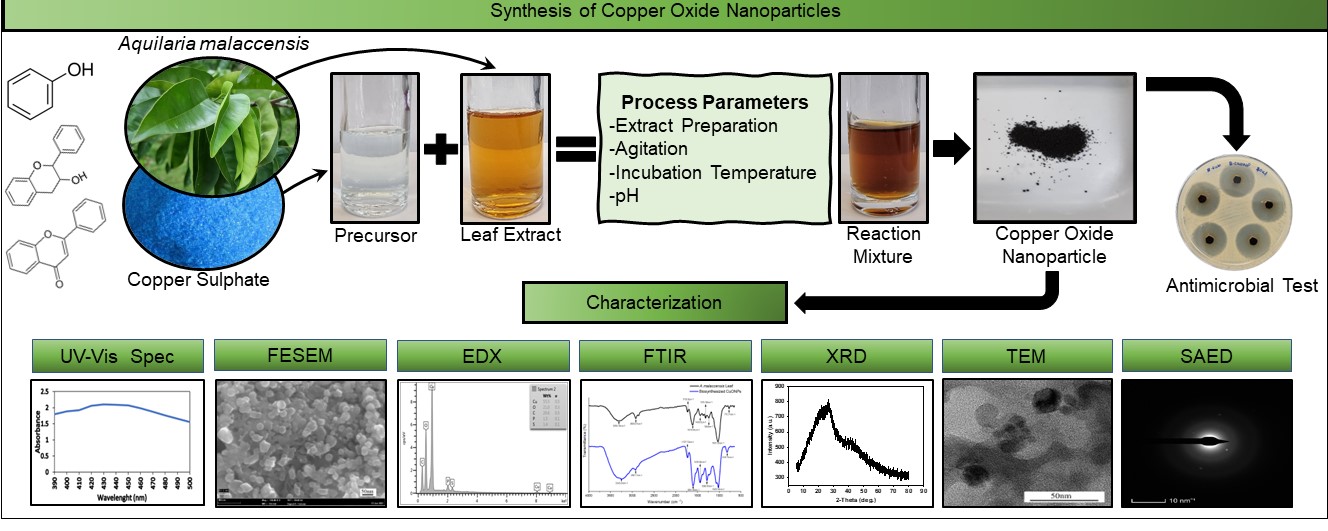
This paper reports the green synthesis of Copper Oxide
nanoparticles (CuONPs) using Aquilaria malaccensis (agarwood) leaf
extract. The main objective of this study was to evaluate the potential of
using A. malaccensis leaf extract as a biogenic medium to generate
CuO NPs with antimicrobial potential. The procedure employed was to add 5 mM
copper sulfate (CuSO4.5H2O) as the precursor to A. malaccensis
leaf extract to study the generation of CuO NPs under different incubation
conditions such as methods of crude extract preparation, precursor
concentration and incubation temperature. The results demonstrated that the
boiled leaf extract reacted with 5 mM CuSO4.5H2O at pH6
and incubated under non-shaking conditions at 70 °C, resulting in a high rate
of CuO NPs formation and depicting a UV absorbance peak of 430 nm. Green
synthesized CuO NPs were characterized using field emission scanning electron
microscopy (FESEM) and energy-dispersive X-ray spectroscopy (EDX),
Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and
transmission electron microscopy (TEM). FESEM and TEM revealed that the
nanoparticles are mainly spherical, ranging from 6 to 32 nm. Antimicrobial
studies showed that 20 µL and 40 µL of 70 µg/µL CuO NPs displayed potent
inhibition towards Gram-positive bacteria Bacillus subtilis, with the
average zone of inhibition measuring 24.43 ± 0.10 mm and 27.31 ± 0.13 mm,
respectively.
Antimicrobial; Aquilaria malaccensis; Copper oxide nanoparticles; Green synthesis; Phytochemical
Copper is a prominent metal with a wide range of applications due to its
pertinent properties such as electrical, conductivity, optical, catalytic, and
antimicrobial applications compared to other metallic structures. At the
nano-size, copper has been used extensively as gas sensors, catalysts, and
superconductors, in photovoltaic devices such as solar panels, dye removal from
wastewater, and agricultural sectors (Jiang et al., 2015; Sundar
et al., 2018; Sone et al., 2020).
Moreover, copper oxide
nanoparticles (CuO NPs) have excellent antifungal and antimicrobial potential,
thus emerging as a promising tool for many industries, including
The standard routes to generate CuO NPs
entail methods delineated using physical or chemical processes such as
hydrothermal, sol-gel, and solid-state reactions (Sohrabnezhad & Valipour, 2013;
Quirino et al., 2018; Dorner et al., 2019; Muktaridha et al., 2021). Although these
processes can be modulated to produce well-defined nanoparticles of preferred
morphology and size, the toxic solvents and the byproducts threaten the environment.
Additionally, some of these methods incur a high cost of investment due to
their high energy requirements, such as the vapor transport method, which works
well in high temperatures up to 1,400°C (Sabir et al., 2014), and the necessity
to use specialized equipment such as ball grinding (Yadav & Vasu, 2016). On the other hand,
green synthesis, or the use of biomaterials as a reducing agent in the
generation of nanostructures, has emerged as a method of choice since it is
facile, cost-effective, and environmentally safe (Kayalvizhi et al., 2020; Siddiqi and
Husen, 2020). Processes using green
synthesis do not pose high energy requirements or the usage of specialized equipment (Sankar et al., 2014; Rajesh et al., 2018),
leading to a significant reduction in cost. They are also efficient and have
less reaction time (Omar et al., 2020). Work on such processes using plant
extracts needs to be intensified to scale it up for industrial production.
Extracts from plant structures such as leaves, seeds, bark, flowers, and stem
contain phytochemicals that can be used as the bioreducing or capping agent in
generating metallic nanoparticles from their aqueous form. Several plant
extracts such as Carica papaya (Sankar
et al., 2014), Syzygium
aromaticum flower bud (Rajesh et al.,
2018), and Azadirachta
indica leaf (Nagar and Devra, 2018) have been explored as natural reducing
and capping agents for the synthesis of copper nanoparticles. In contrast, the
leaf of Aloe vera (Kumar et al.,
2015) and Annona
muricata (Kayalvizhi
et al., 2020) have
been explored to synthesize CuO NPs. In this study, Aquilaria malaccensis
was introduced as an alternative reducing/capping agent for the synthesis of CuO
NPs. Din et al. (2017) observed that a reactive hydrogen atom
in plant flavonoids reduces Cu2+ to form copper nuclei in the
nucleation of CuO NPs. Therefore, it is assumed that flavonoids in plant
extracts act as reducing agents and play an essential role in the nucleation of
CuO NPs (Ferrando, 2016). Capping agents act as binding
molecules which modulate the surface chemistry of the nanoparticles and shield
them to prevent the over-growth of the nanoparticles, which causes
agglomeration (Javed et al., 2020).
Aquilaria malaccensis is rich in phytochemicals such as flavonoids, alkaloids, squalene, saponins, steroids, terpenoids, tannins, n-hexadecanoic acid, tetramethyl-2-hexadecane-1-ol, octadecatrienoic acid and phenols, which has the potential to be utilized as reducing and capping agents in the synthesis of metal nanoparticles (Khalil et al., 2013; Buniyamin et al., 2021). The presence of different phytochemicals is important because flavonoids can undergo a tautomeric transformation and release reactive hydrogen atoms. This mechanism was discovered while reducing metal ions into metal nanoparticles (Singh et al., 2017). Whereas phenolic compound has hydroxyl and ketone groups which help to bind and chelate metal (Singh et al., 2017). In addition, glucose, fructose, and amino acids can reduce metal ions differently (Panigrahi et al., 2004). Hence, the richness of phytochemicals in the plant extract is essential in synthesizing nanoparticles. A previous study has shown that leaf extract of A. malaccensis has been explored in synthesizing tin oxide nanoparticles via the reduction process (Buniyamin et al., 2021). The study’s novelty is that A. malaccensis leaf extract was used to explore the synthesis of CuO NPs without using expensive techniques or toxic chemicals. Therefore, the objective of this study was to study the potential of A. malaccensis leaf extract as a reducing agent in the fabrication of CuO NPs and to characterize these nanostructures using standard techniques such as UV-Visible spectrophotometry, FTIR, XRD, EDX, FESEM, and TEM. The capability of CuO NPs to inhibit bacterial growth was also investigated.
2.1. Collection of Plant Samples and Extract
Preparation
1000 g of A.
malaccensis leaves were collected in the garden of Nilai University, Negeri
Sembilan, Malaysia (2°48'59.2"N 101°46'05.2"E). The leaves were
washed with tap water and then distilled water to remove tiny dust particles
and dehydrated in the oven at 60 °C for two days. The dried leaves were crushed
into a fine powder using a mechanical blender (Xinganbangle, China) and
refrigerated at 4 °C. Leaf extract was prepared by adding 2 g of powdered leaf
samples into 100 ml of distilled water and boiling for 10 minutes. This mixture
was cooled to room temperature and then centrifuged (UNIVERSAL 16R, Model
LWB-122D) at 4000 rpm for 10 minutes to separate the particulate and the
extract. The supernatant was transferred to a new tube and centrifuged for 20
minutes. The purified supernatant was stored in the refrigerator at 4 °C to
synthesize CuO NPs.
2.2. The Influence of Process
Parameters on the Green Synthesis of CuO NPs
CuO NPs were
synthesized by mixing an equal volume of aqueous leaf extract with 5 mM aqueous
CuSO4.5H2O (Bendosen, Malaysia) as a precursor. A color
change from light brown to dark brown/reddish brown indicated the formation of CuO
NPs. The reaction mixture was centrifuged at 15,000 rpm for 20 minutes, and the
pellet was washed three times using distilled water. The pellet was then
crushed after drying in the oven at 60 °C for 2 h. Powdered CuO NPs were
calcinated (Lindberg/Blue M, United States) at 600 °C for 3 h. The influence of
various process parameters on the synthesis of CuO NPs was observed at
different concentrations (5 mM and 10 mM) of the precursor, CuSO4.5H2O,
extract preparation through boiling for 10 minutes or heating at 70 °C for 20
minutes, agitation of the reaction mixture and pH (pH 6, 9 and 12) of the
reaction mixture. The schematic illustration of the green synthesis of CuO NPs
is depicted in Figure 1.
Figure 1
Schematic illustration of green synthesis of CuO NPs
2.3. Characterization of Green Synthesized CuO NPs
The optical properties of CuO NPs were
characterized using a UV-visible absorption spectrophotometer (HALO RB-10
Dynamica, Australia). Fourier transform infrared spectroscopy (FTIR)(Perkin
Elmer, Spectrum 400, United States), X-ray diffraction (XRD) (D8 Advance,
Bruker AXS, Germany), Field emission scanning microscopy (FESEM)(ZEISS, SUPRA
55VP, Germany) with energy-dispersive X-ray spectroscopy (EDX)(AZtecEnergy EDX
80 mm X-Max SDD detector, United Kingdom), and transmission electron microscopy
(TEM)(Talos L120C, ThermoFisher, United States) to verify the generation of CuO
NPs and to assess its morphology, size, and elemental composition.
2.4. Antimicrobial Activity of CuO
NPs
The agar well diffusion method evaluated antimicrobial activity against Gram-positive (Bacillus subtilis—ATCC 6051) bacteria. Nutrient agar plates were inoculated using a cotton swab dipped in 106 CFU McFarland Standard bacterial suspensions. Wells of 6 mm diameter were bored in the inoculated plates using a sterile borer. These wells were then loaded with 40 ul green synthesized and commercial CuO NPs (Copper (II) oxide, 30-50 nm, Alfa Aesar) (70ug/ml). Control wells were filled with 40 µL of A. malaccensis leaf extract and ampicillin (Santa Cruz, California) (70 ug/ul) as the positive control. These plates were incubated at 37 °C for 24 h, and antibacterial activities were evaluated by measuring the inhibition zone diameter around the wells.
3.1. Optimization of
Different Parameters in Biosynthesizing CuO NPs
3.1.1.
Preparation of Crude Leaf Extract of A. Malaccensis
Leaf
extract preparation under different conditions was explored in this section.
First, adding 5 mM CuSO4.5H2O to leaf extract, which was
boiled for 10 minutes, showed intense color change compared to heating the leaf
extract at 70 °C for 20 minutes. The leaf samples used in this study were powdered
to increase the surface contact between the sample and the solvent. Although
the surface contact of the leaf powder sample was achieved by crushing yet,
boiling the leaf extract at 100 °C for 10 minutes is assumed to aid in the
rapid release of phytochemicals, causing the increase in color intensity of the
reaction mixture compared to heating at 70 °C for 20 minutes. UV-Vis analysis
showed a uniform size distribution of the synthesized CuO NPs using boiled leaf
extract than the heating preparation. However, both leaf extracts validate the
peak at 420 nm.
3.1.2. Agitation of the Reaction
Mixture
Agitation
of the reaction mixture is demonstrated to influence the green synthesis of CuO
NPs using A. malaccensis aqueous leaf extract. The intensity of color
change in the reaction mixture increased in the reaction mixture, which was
left without agitation compared to agitating the reaction mixture. An increase
in the intensity of the brown pigmentation in the reaction mixture correlates
to the rise in the formation of CuO NPs, stipulating that non-agitation
conditions were more conducive for the green synthesis of CuO NPs. The reaction
mixture without agitation demonstrated a higher absorbance intensity than the
agitated reaction, suggesting a higher rate of CuONP formation.
3.1.3. Concentration
of the Precursor
CuO NPs formation depended on the concentration of the precursor, aqueous CuSO4.5H2O, used for the reaction mixture. The reaction mixture was observed at 5 mM and 10 mM CuSO4.5H2O. The resulting reaction mixture showed that when 10 mM precursor was used, CuO NPs aggregated by the first 20 minutes of reaction. Aggregation of nanoparticles results in bulk form and may show different diameters and particle size distribution (Dang et al., 2011a). According to Dang et al. (2011b), the copper particles aggregate during nuclei formation to reduce the total surface energy. This aggregation may result from attractive Van Der Waals forces between the crystals formed. Also, another reported article stated that an increase in precursor from 6 mM to 7.5 mM concentration led to increasing particle size, which significantly resulted in aggregation and growth of particle size (Nagar and Devra, 2018). On the other hand, a study from Kumar et al. (2015) proves that copper nitrate at a concentration of 10 mM reacts with leaf extract of Aloe vera leaf extract, resulting in overlapping and aggregation of smaller particles. Hence, copper sulfate precursor at 10 mM became very hard to characterize using UV-Vis spectrophotometer due to the growth of precipitates; however, the precursor concentration of 5 mM was stable without any precipitation observed for up to 3 weeks.
3.1.4. Incubation Temperature
of the Reaction Mixture
The incubation temperature of the reaction
mixture is known to influence the formation of metallic nanoparticles in green
synthesis. Nagar
and Devra (2018) showed that the conversion rate of Cu2+
to CuO NPs gradually increased as the temperature rose from 60 to 85 °C due to
a rapid nucleation rate. The UV-Vis spectrophotometer results recorded showed
that the formation rate of CuO NPs doubled at 70 °C compared to room
temperature, confirming the postulation that an increase in reaction
temperature increases the reaction rate reducing Cu2+ metal ions to
form the nuclei of the CuO NPs (Joshi et al., 2019).
3.1.5. The pH of the Reaction
Mixture
Green synthesis of CuO NPs using aqueous
extract of A. malaccensis leaf extract was examined over a broad pH
range (9 and 12). As reflected in the absorbance, changes in pH highly affected
the surface plasmon resonance (SPR) of the CuO NPs. It was observed that the
absorbance peak shifted from 430 nm at pH 6 to 340 nm in alkaline pH of pH9 and
pH12, confirming the observation made by Thamer et al. (2018).
According to Nagar
and Devra (2018), pH is essential in the synthesis of
nanoparticles, and changes in pH directly affect the rate of synthesis and the
morphology of nanoparticles. A change in pH affects the charges of
biomolecules, affecting their stabilizing and capping ability. It was
discovered that nanoparticles were not formed in acidic conditions such as pH
4.7 due to the suppressing effect of acidic pH that inactivated biomolecules.
Raising the pH to pH 6 and pH 6.6 resulted in the synthesis of more small-sized
nanoparticles due to the availability of functional groups in biomolecules
responsible for copper binding. Even higher pH was discovered to be efficient in
the synthesis of nanoparticles; however, nanoparticles tend to form large size
nanoparticles due to agglomeration. Hulkoti and Taranath (2014) observed that the pH
of the reaction mixture influences the size, shape, and composition of CuO NPs.
Figure 2
UV-Vis absorption spectra of green synthesized CuO NPs
3.2. Characterization of CuO
NPs
3.2.1.
UV Spectroscopy
The addition of the A. malaccensis
aqueous leaf extract to the precursor, aqueous CuSO4.5H2O,
resulted in a color change from light brown to dark brown in the reaction
mixture after 2 h, which intensified after 24 h. The indication of the
formation of CuO NPs in the reaction mixture was confirmed through an SPR peak
observed at a wavelength of 430 nm (Figure 2) using UV spectrophotometry after
24 h incubation based on the reports of Thamer et al. (2018) and Naika et al. (2015),
which reported SPR peaks located at wavelengths of 392 nm and 415 nm,
respectively as indicative of the formation of CuO NPs.
3.2.2. Field Emission
Scanning Electron Microscopy and Dispersive X-ray Spectrograph
Figure 3 (a)
FESEM image, (b) Histogram of the particle size distribution based on FESEM
image, (c) EDX spectrum, and (d) FTIR of biosynthesized CuO NPs
3.2.3. FTIR Analysis
Figure 4 (a) XRD pattern of biosynthesized CuO NPs (b) TEM image of CuO NPs, (c)
Histogram of the particle size distribution based on TEM image, and (d)
Intermittent dots on SAED
3.2.4. X-ray Diffraction
Analysis
XRD
analysis exposed the crystalline nature of the CuO NPs, as shown in Figure 4a.
XRD micrograph showed small distinct diffraction peaks at 21.74, 32.95, and
42.14. These corresponding peaks represent (100), (110), and (200) of CuO NPs
primitive structure. The grain size of CuO NPs formed in the bio-reduction
process was measured using the Debye-Scherrer formula (D= k?/? cos ?), where D
is the average crystalline size, k represents constant 1, ‘?’ is the wavelength
of x-ray source (0.15406 nm), ? is the angular line full width at half maximum
(FWHM) intensity in radians and ‘?’ the Bragg’s angle. The XRD pattern showed
that the average crystallite size was 1.08 nm.
3.2.5. Transmission Electron
Microscopy
TEM analysis further confirmed the
crystalline nature of the green synthesized CuO NPs, found as clusters due to
aggregation, as shown in Figure 4b. The CuO NPs did not show a uniform
distribution and ranged from 6nm to 22 nm (Figure 4b) in size. Particle size
analysis from the TEM micrograph was done using ImageJ and OriginPro 2021 and
shown in a histogram (Figure 4c), where the average particle size obtained was
7 nm. Intermittent dots on Selected Area Electron Diffraction (SAED) on the
concentric circle confirmed the crystalline nature of green synthesized CuO NPs,
as depicted in Figure 4d. Similar results were reported by Mali et al. (2019)
and Nabila
and Kannabiran (2018). On a microscopic scale,
the nanoparticles showed good dispersion in bio-reduced aqueous solution, which
is explained through the results of the SAED.
3.2.6. Antibacterial
Activity of the CuO NPs
Table
1 shows the mean diameter of inhibition zones (in mm) for three replicates
containing CuO NPs suspension. The negative control used were A. malaccensis
leaf extract, CuSO4.5H2O, and commercial CuO NPs. A. malaccensis
leaf extract showed no inhibition effect due to no formation of clear inhibited
zones on bacteria B. subtilis. However, 100mM CuSO4.5H2O
showed an inhibition effect (26.03 ± 0.19 mm). Increasing the concentration of
CuSO4.5H2O to 200 mM resulted in an increased diameter of
the inhibition zones, 31.83 ± 0.29 mm. Copper has been utilized as an
alternative antibacterial agent due to its novel and promising effect on
nosocomial infections (Benhalima et al., 2019). Copper can also
produce reactive oxygen species (ROS), inactivate enzymes, modify cell walls,
and alter nucleic acid synthesis, significantly inhibiting nosocomial
infections' growth (Gant et al., 2007). Hence, the presence of the
Cu element in CuSO4. 5H2O inhibits the growth of bacteria
such as B. subtilis (gram-positive) in the present study. Increasing the
treatment concentration will also increase the availability of copper ions to
induce ROS and other activity towards the bacteria; therefore, the inhibition
effect on the bacteria such as B. subtilis was increased as the
concentration was also increased. On the other hand, 20 µL of commercial CuO
NPs can inhibit B. subtilis (26.60 ± 0.47 mm). A similar observation was
found using biosynthesized CuO NPs where an inhibition zone of 24.43 ± 0.10 mm
and 27.31 ± 0.13 mm, respectively, were obtained when 20 µL and 40 µL of
biosynthesized CuO NPs on B. subtilis. These results may indicate that
the CuO NPs synthesized using A. malaccensis leaf extract are less toxic
than commercial CuO NPs presumably produced by chemical methods. The reduced
toxicity could be due to the smaller size of CuO NPs generated through green
processes (size: 6 -32nm) compared to chemically synthesized ones (30 to 50nm),
as explained by Letchumanan et al. (2021).
Table 1 Antibacterial activity of
biosynthesized CuO NPs using the well diffusion method
|
Treatment |
Inhibition (mm) ± SE. |
|
Leaf Extract (20 µL) |
0a |
|
CuSO4.5H2O
(100mM) |
26.03 ± 0.19b |
|
CuSO4.5H2O
(200mM) |
31.83 ± 0.29c |
|
Commercial CuO NPs (70 µg / µL, 20 µL) |
26.60 ± 0.47bd |
|
Commercial CuO NPs (70 µg / µL, 40 µL) |
29.98 ± 0.24e |
|
Biosynthesized CuO NPs
(70 µg / µL, 20 µL) | |
|
Biosynthesized CuO NPs
(70 µg / µL, 40 µL) | |
|
Ampicillin (70 µg / µL, 20 µL) |
40.28 ± 0.16h |
A facile,
cost-effective, and sustainable synthesis of CuO NPs was achieved using the
leaf extract of A. malaccensis as a reducing agent. Studies on reaction
conditions showed that leaf extract prepared by boiling for 10 minutes and
incubated under the non-shaking condition with the precursor at 70 °C and pH 6
resulted in rapid and increased formation of CuO NPs. The UV-Visible
spectrophotometry analysis revealed the SPR peak at 430 nm. FTIR result
confirms the phytochemicals from A. malaccensis leaf extract responsible
for the synthesis of CuO NPs. XRD spectra confirmed the crystalline nature of CuO
NPs with an average crystallite size of 1.08 nm. FESEM, TEM, and EDX revealed
the presence of spherical CuO NPs with an average particle size of 6 to 32 nm.
This method proves that CuO NPs can be synthesized without toxic solvents or
high-cost equipment. Antimicrobial studies showed that these CuO NPs at the
concentration of 20 and 40 µL of 70 µg/µL could inhibit the growth of
Gram-positive B. subtilis with an average inhibition zone of 24.43 ±
0.10 mm and 27.31 ± 0.13 mm. Further studies should be conducted to determine
the antimicrobial potential of these nanoparticles in a broader range of
microbial pathogens.
The authors would like to thank Chris Izaak Jones
and Cornelius Berani Anak Paul Ringo for their assistance in the experiments
and Nilai University for providing the necessary facilities. This study was
supported by the Research and Innovation of Private Higher Education Network
(RIPHEN) in the Digital Futures project (MMUE/200003) framework coordinated by
Koo Ah-Choo, Multimedia University, Malaysia, and the Malaysian Institute of
Retirement Management (MIRM).
Benhalima, L., Amri, S., Bensouilah, M.,
Ouzrout, R., 2019. Antibacterial Effect of Copper Sulfate Against Multi-Drug
Resistant Nosocomial Pathogens Isolated from Clinical Samples. Pakistan
Journal of Medical Sciences, Volume 35(5), pp. 1322–1328
Buniyamin, I., Akhir, R.M., Asli, N.A.,
Khusaimi, Z., Mahmood, M.R., 2021. Biosynthesis of SnO2 Nanoparticles by
Aqueous Leaves Extract of Aquilaria Malaccensis (agarwood). In: IOP
Conference Series: Materials Science and Engineering, Volume 1092(1), p.
012070
Chalandar, H.E., Ghorbani, H.R., Attar, H.,
Alavi, S.A., 2017. Antifungal Effect of Copper and Copper Oxide Nanoparticles
Against Penicillium on Orange Fruit. Biosciences Biotechnology
Research Asia, Volume 14(1), pp. 279–284
Dang, T.M.D., Le, T.T.T., Fribourg-Blanc, E.,
Dang, M.C., 2011a. The Influence of Solvents and Surfactants on the Preparation
of Copper Nanoparticles by a Chemical Reduction Method. Advances in Natural
Sciences: Nanoscience and Nanotechnology, Volume 2(2), p. 025004
Dang, T.M.D.,
Le, T.T.T., Fribourg-Blanc, E., Dang, M.C., 2011b. Synthesis and Optical
Properties of Copper Nanoparticles Prepared by a Chemical Reduction Method. Advances
in Natural Sciences: Nanoscience and Nanotechnology, Volume 2(1), p. 015009
Din, M.I., Arshad, F., Hussain, Z., Mukhtar,
M., 2017. Green Adeptness in the Synthesis and Stabilization of Copper
Nanoparticles: Catalytic, Antibacterial, Cytotoxicity, and Antioxidant
Activities. Nanoscale Research Letters, Volume 12(1), pp. 1–15
Dörner, L., Cancellieri, C., Rheingans, B.,
Walter, M., Kägi, R., Schmutz, P., Kovalenko, M.V., Jeurgens, L.P., 2019.
Cost-effective Sol-gel Synthesis of Porous CuO Nanoparticle Aggregates with
Tunable Specific Surface Area. Scientific Reports, Volume 9(1), pp. 1–13
Ferrando, R., 2016. Chapter 3-Synthesis and
Experimental Characterization of Nanoalloy Structures. Structure and
Properties of Nanoalloys, Volume 10, pp. 47–74.
Gant, V.A., Wren, M.W., Rollins, M.S.,
Jeanes, A., Hickok, S.S., Hall, T.J., 2007. Three Novel Highly Charged
Copper-based Biocides: Safety and Efficacy Against Healthcare-Associated
Organisms. Journal of Antimicrobial Chemotherapy, Volume 60(2), pp. 294–299
Helmiyati, Anggraini, Y., 2019.
Nanocomposites Comprising Cellulose and Nanomagnetite as Heterogeneous
Catalysts for the Synthesis of Biodiesel from Oleic Acid. International
Journal of Technology, Volume 10(4), pp. 798–807
Hulkoti, N.I., Taranath, T.C., 2014.
Biosynthesis of Nanoparticles Using Microbes: A Review. Colloids and
Surfaces B: Biointerfaces, Volume 121, pp. 474–483
Javed, R., Zia, M., Naz, S., Aisida, S.O.,
Ao, Q., 2020. Role of Capping Agents in the Application of Nanoparticles in
Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. Journal
of Nanobiotechnology, Volume 18(1), pp. 1–15
Jiang, T., Wang, Y., Meng, D., Yu, M., 2015.
Facile Synthesis and Photocatalytic Performance of Self-assembly CuO
Microspheres. Superlattices and Microstructures, Volume 85, pp. 1–6
Joshi, A., Sharma, A., Bachheti, R.K., Husen,
A., Mishra, V.K., 2019. Plant-mediated Synthesis of Copper Oxide Nanoparticles
and Their Biological Applications. Nanomaterials and Plant Potential,
Volume 2019, pp. 221–237
Kayalvizhi, S., Sengottaiyan, A.,
Selvankumar, T., Senthilkumar, B., Sudhakar, C., Selvam, K., 2020. Eco-friendly
Cost-effective Approach for Synthesis of Copper Oxide Nanoparticles for
Enhanced Photocatalytic Performance. Optik, Volume 202, p. 163507
Khalil, A.S., Rahim, A.A., Taha, K.K.,
Abdallah, K.B., 2013. Characterization of Methanolic Extracts of Agarwood
Leaves. Journal of Applied and Industrial Sciences, Volume 1(3), pp. 78–88
Kumar, P.P.N., Shameem, U., Kollu, P.,
Kalyani, R.L., Pammi, S.V.N., 2015. Green Synthesis of Copper Oxide
Nanoparticles Using Aloe Vera Leaf Extract and its Antibacterial
Activity Against Fish Bacterial Pathogens. BioNanoScience, Volume 5(3), pp. 135–139
Letchumanan, D., Sok, S.P., Ibrahim, S.,
Nagoor, N.H., Arshad, N.M., 2021. Plant-based Biosynthesis of Copper/Copper
Oxide Nanoparticles: An Update on Their Applications in Biomedicine,
Mechanisms, and Toxicity. Biomolecules, Volume 11(4), pp. 1–27
Mali, S.C., Raj, S., Trivedi, R., 2019.
Biosynthesis of Copper Oxide Nanoparticles Using Enicostemma Axillare
(Lam.) Leaf Extract. Biochemistry and Biophysics Reports, Volume 20, p.
100699
Maqbool, Q., Iftikhar, S., Nazar, M., Abbas,
F., Saleem, A., Hussain, T., Kausar, R., Anwaar, S., Jabeen, N., 2017. Green
Fabricated CuO Nanobullets via Olea Europaea Leaf Extract Shows
Auspicious Antimicrobial Potential. IET Nanobiotechnology, Volume 11(4),
pp. 463–468
Muktaridha, O., Adlim, M., Suhendrayatna, I.,
Bakar, N.H.H.A., 2021. Photocatalytic Degradation of Skim-Latex-Vapor Odor
Using Iron-Doped Zinc Oxide. International Journal of Technology, Volume
12(4), pp. 739–748
Nabila, M.I., Kannabiran, K., 2018.
Biosynthesis, Characterization and Antibacterial Activity of Copper Oxide
Nanoparticles (CuO NPs) from Actinomycetes. Biocatalysis and Agricultural
Biotechnology, Volume 15, pp. 56–62
Nagar, N., Devra, V., 2018. Green Synthesis
and Characterization of Copper Nanoparticles Using Azadirachta Indica
Leaves. Materials Chemistry and Physics, Volume 213, pp. 44–51
Naika, H.R., Lingaraju, K., Manjunath, K.,
Kumar, D., Nagaraju, G., Suresh, D., Nagabhushana, H., 2015. Green Synthesis of
CuO Nanoparticles Using Gloriosa Superba L. Extract and Their
Antibacterial Activity. Journal of Taibah University for Science, Volume
9(1), pp. 7–12
Omar, M.S., Sanif, M.N.M., Omar, N.H.S., Ali,
M.H.S.A.H., Taha, H., Mahadi, A.H., Soon, Y.W., Ngaini, Z., Rosli, M.Y.H.,
Usman, A., 2020. Synthesis of Schiff Base Encapsulated ZnS Nanoparticles:
Characterization and Antibacterial Screening. International Journal of
Technology, Volume 11(7), pp. 1309–1318
Osuntokun, J., Onwudiwe, D.C., Ebenso, E.E.,
2017. Biosynthesis and Photocatalytic Properties of SnO2 Nanoparticles Prepared
Using Aqueous Extract of Cauliflower. Journal of Cluster Science, Volume
28(4), pp. 1883–1896
Panigrahi, S., Kundu, S., Ghosh, S., Nath,
S., Pal, T., 2004. General Method of Synthesis for Metal Nanoparticles.
Journal of Nanoparticle Research, Volume 6(4), pp. 411–414
Quirino, M.R., Lucena, G.L., Medeiros, J.A.,
Santos, I.M.G.D., Oliveira, M.J.C.D., 2018. CuO Rapid Synthesis with Different
Morphologies by the Microwave Hydrothermal Method. Materials Research,
Volume 21, p. e20180227
Rajesh, K.M., Ajitha, B., Reddy, Y.A.K.,
Suneetha, Y., Reddy, P.S., 2018. Assisted Green Synthesis of Copper
Nanoparticles Using Syzygium Aromaticum Bud Extract: Physical, Optical
and Antimicrobial Properties. Optik, Volume 154, pp. 593–600
Sabir, S., Arshad, M., Chaudhari, S.K., 2014.
Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and
Applications. The Scientific World Journal, Volume 2014, p. 925494
Sankar, R., Manikandan, P., Malarvizhi, V.,
Fathima, T., Shivashangari, K.S., Ravikumar, V., 2014. Green Synthesis of
Colloidal Copper Oxide Nanoparticles Using Carica Papaya and its
Application in Photocatalytic Dye Degradation. Spectrochimica Acta Part A:
Molecular and Biomolecular Spectroscopy, Volume 121, pp. 746–750
Siddiqi, K.S., Husen, A., 2020. Current Status
of Plant Metabolite-based Fabrication of Copper/Copper Oxide Nanoparticles and
Their Applications: A Review. Biomaterials Research, Volume 24(1), pp. 1–15
Singh, S., Kumar, N., Kumar, M., Agarwal, A.,
Mizaikoff, B., 2017. Electrochemical Sensing and Remediation of 4-Nitrophenol
Using Bio-synthesized Copper Oxide Nanoparticles. Chemical Engineering
Journal, Volume 313, pp. 283–292
Sivaraj, R., Rahman, P.K., Rajiv, P., Salam,
H.A., Venckatesh, R., 2014. Biogenic Copper Oxide Nanoparticles Synthesis Using
Tabernaemontana Divaricate Leaf Extract and its Antibacterial Activity
Against Urinary Tract Pathogen. Spectrochimica Acta Part A: Molecular and
Biomolecular Spectroscopy, Volume 133, pp. 178–181
Sohrabnezhad, S., Valipour, A., 2013.
Synthesis of Cu/CuO Nanoparticles in Mesoporous Material by Solid State
Reaction. Spectrochimica Acta Part A: Molecular and Biomolecular
Spectroscopy, Volume 114, pp. 298–302
Sone, B.T., Diallo, A., Fuku, X.G.,
Gurib-Fakim, A., Maaza, M., 2020. Biosynthesized CuO Nano-platelets: Physical
Properties and Enhanced Thermal Conductivity Nanofluidics. Arabian Journal
of Chemistry, Volume 13(1), pp. 160–170
Sundar, S., Venkatachalam, G., Kwon, S.J.,
2018. Biosynthesis of Copper Oxide (CuO) Nanowires and Their Use for the
Electrochemical Sensing of Dopamine. Nanomaterials, Volume 8(10), pp.
823–839
Thamer, N.A., Muftin, N.Q., Al-Rubae, S.H.N.,
2018. Optimization Properties and Characterization of Green Synthesis of Copper
Oxide Nanoparticles Using Aqueous Extract of Cordia Myxa L. Leaves. Chemistry
- An Asian Journal, Volume 30(7), pp. 1559–1563
Yadav, S.K., Vasu, V., 2016. Synthesis and
Characterization of Copper Nanoparticles, Using Combination of Two Different
Sizes of Balls in Wet Ball Milling. International Journal of Emerging Trends
in Science and Technology, Volume 3(4), pp. 2348–9480
