A Comparison Study of Nickel Laterite Reduction using Coal and Coconut Shell Charcoal: A FactSage Simulation
Corresponding email: bayupetrus@ugm.ac.id
Published at : 04 Apr 2023
Volume : IJtech
Vol 14, No 2 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i2.5808
Putera, A.D.P., Warmada, I.W., Amijaya, D.H., Astuti, W., Sukadana, I.G., Petrus, H.T.B.M., 2023. A Comparison Study of Nickel Laterite Reduction using Coal and Coconut Shell Charcoal: A FactSage Simulation. International Journal of Technology. Volume 14(2), pp. 267-275
| Andreas Diga Pratama Putera | 1. Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Yogyakarta 55281, Indonesia, 2. Fluid and Pr |
| I Wayan Warmada | 1. Department of Geological Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Yogyakarta 55281, Indonesia, 2. Unconventional Georesources Research Group, Faculty of Engi |
| Donatus Hendra Amijaya | 1. Department of Geological Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Yogyakarta 55281, Indonesia, 2. Unconventional Georesources Research Group, Faculty of Engi |
| Widi Astuti | Research Center for Mining Technology, National Research and Innovation Agency (BRIN), Jl. Ir. Sutami, Km. 15, Tanjung Bintang, South Lampung, Lampung Province, Indonesia |
| I Gde Sukadana | Research Center for Nuclear Fuel Cycle and Radioactive Waste TechnologyNational Research and Innovation Agency of Indonesia (BRIN), Gedung 57 Kawasan Nuklir Pasar Jumat, Jl. Lebak Bulus Raya - Pasar J |
| Himawan Tri Bayu Murti Petrus | 1. Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Yogyakarta 55281, Indonesia, 2. Unconvention |
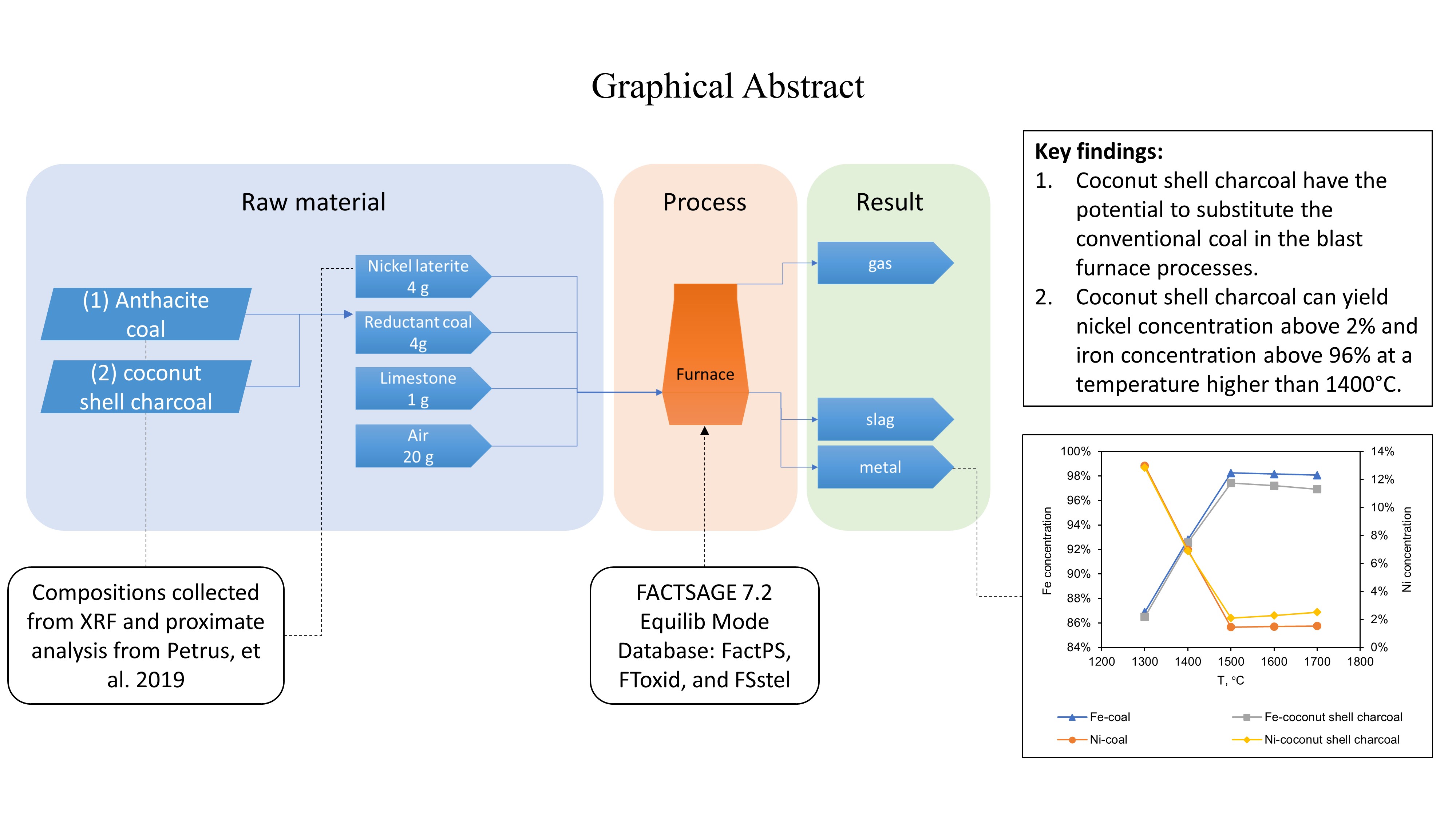
Replacing metallurgical coke with greener material is a long-term challenge in pyrometallurgy. With the fact of biomass abundance in Indonesia, the usage of bio-char as a replacement for coke resulted from coal has been an intensive study. This study compared the performance between anthracite coal and coconut shell charcoal for nickel laterite reduction using the Equilibrium module on FactSage software simulation. The simulation was done by inputting a 4:4:1:20 weight ratio of nickel laterite ore, reductant (coal or coconut shell charcoal), lime and air, respectively. The temperature studied was 1300°C to 1700°C, and the pressure was atmospheric. The result shows that coconut shell charcoal has the potential to substitute anthracite coal as a reductant material in blast furnace processes. Anthracite coal consistently gives better results on the metal phase product with higher liquid metal yield and higher nickel and iron content. However, the coconut shell charcoal posseses the potential as a substitute material with temperatures above 1400°C regarding to the higher nickel concentration compared to anthracite coal. The resulting nickel and iron concentrations can reach above 2% and 96%, respectively, which is the required characteristics of a nickel pig iron product.
FactSage; Nickel; Pyrometallurgy
Nickel
could be a
transition
component that has properties of ferrous and nonferrous metal properties (Kim et al., 2010). Nickel store is
affiliated with the press (nickel laterite) or sulfur (nickel sulfide). Almost
58% of nickel request is provided by sulfide metals, in spite of the fact that
78% of nickel stored lies in laterite minerals (Petrus et al., 2019). However, as sulphidic ores were continuously exploited in recent years, the sources
began to become scarce, and underground mining has been introduced.
Consequently, the exploitation cost was rising, especially the labor cost. On
the contrary, the mining activity of laterite deposits is considerably shallow
(usually less than 50 meters). So, much concern has been concentrated on using low-grade nickel ore
(especially those containing <2.0 wt.% nickel), such as laterites (Petrus
et al., 2019).
About 12% of the world's
nickel laterite resources are stored in Indonesia, mostly in the form of
laterites (Rasyid and Petrus, 2016). There are two kinds of laterite, namely limonite and
saprolite. Limonite is low-nickel content laterite (around 0.8-1.5% Ni-mass), and
saprolite is a rich-nickel content (more than 1.5-3% Ni-mass) (Zhou et
al., 2017). Both
hydrometallurgical and pyrometallurgical processes can be used to extract
nickel from the laterites. However, due to its high nickel content, saprolite
ore is better processed by pyrometallurgy (Minister of Energy and Mineral
Resources Republic of Indonesia, 2013; Li, Wang, and Wei, 2011). In the
pyrometallurgical process there are usually three unit
operations, namely roasting, smelting, and converting. The reduction process
consumes carbon-based reductants, usually coke, and produces a huge amount of
carbon dioxide. This process is highly energy-consuming and not environmentally
friendly. Concerning carbon dioxide emission, replacing coke using bio-reductant has
been an interesting issue to be studied.
The studies conducted to
explore the possibility of using bio-reductants in the process, while paying
attention to certain parameters, have been limited. Chen et al. (2015) suggested that bio-coal
reductants can be used to reduce the major phase in the limonitic laterite ore
(Fe1.833(OH)0.5O2.5, and Fe2SiO4)
into a metal phase, such as Fe, Fe0.64Ni0.36. Yunus et al. (2014) suggested that bio-char derived from empty
fruit bunches can enhance the magnetic properties of goethite-rich iron ore via
a temperature-dependent sequential reduction process involving hematite
(Fe2O3), magnetite (Fe3O4), and wustite (FeO). Wanta et al. studied
the nickel extraction from nickel laterite ore using citric acid leaching (Wanta et
al., 2022), but the
big-scale metallurgical process still prefers high-temperature slag treatment (Chen et
al., 2019). Petrus et
al. studied the kinetics and mechanism of saprolitic nickel laterite
reduction using palm kernel shell charcoal under 1000°C and shows the promising
result of the bio-based charcoal utilization in the process (Petrus
et al., 2022).
Following Indonesia's target
to export a minimum nickel content of 4.0%, developing a nickel laterite
processing plant in the country is necessary. To support the idea, a study
confirms that Ni content in Fe-Ni alloy from lateritic sources can reach 4.5% (Citrawati,
Dwiwandono, and Firmansyah, 2020). The latest research concerning the phase transformation and kinetics
study uses coconut shell and lamtoro charcoal as reductants and proves that
biomass-based charcoal could be a good substitute for conventional coal in the
roasting process of nickel laterite. Both studies yield magnetite (Fe3O4)
with identical kinetics parameters to conventional coal, leading to a good step
for biomass-based charcoal to substitute conventional coal (Petrus
et al., 2017; Putera et al., 2017). It has been widely
known that carbothermic reduction of nickel laterite ore for nickel pig iron
production is an exceptionally dirty process as it requires a vast energy and
emits huge amounts of carbon to the atmosphere. Blast furnace smelting is the
oldest method that is later used to process nickel laterite to produce 2-5% Ni.
Rotary-kiln electric furnace (RKEF) is a much better process that harnesses the
electric current to melt the nickel laterite relatively quicker that
harnesses the electric current to melt the nickel laterite in a relatively
quick time and produces 20-40% Ni in the final product. The krupp-Renn process
is addressed to the low-grade iron ore and able to produce approximately 23%
Ni. All current nickel laterite processes, including blast furnace, RKEF, and
Krupp-Renn, harness high temperatures. In addition, the blast furnace and
Krupp-Renn process use metallurgical coke, anthracite, and limestone (Rao et
al., 2013).
Therefore, this research studies the potential of an eco-friendly material,
charcoal from coconut shells, to substitute the conventional coke commonly used
in the mentioned processes.
The current work aims to provide a
thermodynamical analysis using FactSage 7.2 of nickel laterite reduction
process using conventional coal and coconut shell charcoal. The software offers
a benefit to predicting the result of high temperature experiments through
complex thermodynamical database calculation (Islam, 2015). Coconut shell charcoal is selected due to
its unique characteristics, such as being rich in carbon, environmentally
friendly, and cheap (Purnomo et al., 2017). The observed yield of the process will be
iron and nickel concentrations. The current study is a continuation of the
previous study by Petrus et al. (2019),
which was limited to 1000°C.
2.1. Raw material characterization
The type of nickel laterite used in this
research is limonitic laterite. The reductants are anthracite coal collected
from Lembaga Ilmu Pengetahuan Indonesia (LIPI) and coconut shell
charcoal collected from a local market. The composition of the limonitic laterite,
anthracite coal and coconut shell charcoal has been studied by Petrus et al.,
as shown in Tables 1 to 3.
Table 2 Reductant material compositions (Petrus et al., 2019)
|
Composition %wt Reductant |
Moisture |
Volatile Matter |
Ash |
Fixed Carbon |
|
Anthracite coal |
2.3 |
7.4 |
2.5 |
87.9 |
|
Coconut shell charcoal |
6 |
13.2 |
3.7 |
77.2 |
Table 3 Ash
composition for different reductant types (Petrus
et al., 2019)
|
Ash |
SiO2 |
Al2O3 |
Fe2O3 |
CaO |
MgO |
Na2O |
K2O |
SO3 |
|
Anthracite coal |
50.9 |
34.9 |
3.5 |
2.4 |
1.4 |
0.2 |
0.3 |
0.4 |
|
Coconut shell charcoal |
6.8 |
ND |
0.7 |
9.4 |
10.7 |
13.4 |
40.8 |
14.1 |
Figure 1
Ellingham diagram of selected elements (C, Fe, Ni, and Cr)
2.2.
Process simulation
The process of pig iron making is illustrated in Figure 2. Laterite, reductant, coke, and slag are fed into the blast furnace. Silica and alumina are naturally present in the laterite and must be removed in the slag phase, which commonly uses lime (CaO). This study's selected mass ratio is 4:4:1 for the laterite ore, reductant, and lime. A 1:1 ratio between the ore and coal is assumed to be enough for the reduction process, whereas 4:1 ratio between the coal and lime is the generally accepted practice in the industry (Chakraborty, 2014). In addition, excess hot air (5 times of laterite mass) is fed to the system.
Figure 2 Process
illustration
Tables
2 and 3 clearly show that alumina and silica are present in the system. The
addition of CaO has the potential to reduce slag's melting point. From the
explanation above, the major concentration for the slag will be the CaO-SiO2-Al2O3
system, and the slag's composition will be 53%CaO-14%SiO2-33%Al2O3
(%wt) and 56%CaO-11%SiO2-33%Al2O3 (% wt) for
the system with anthracite coal and coconut shell charcoal, respectively.
Before the temperature of the simulation is determined, the minimum melting
point of the slag must be predicted first.
Figure 3 Phase
diagram for CaO-SiO2-Al2O3 system at 1500°C
and 1 atm
2.3.
FactSage simulation input
FactSage is a simulation software
introduced in 2001, which is the combination of FACT-Win and ChemSage
thermochemical packages that were founded 25 years earlier. In this study,
FactSage version 7.2 is used. FactSage consists of information, database and
calculation modules most beneficial to chemical and physical metallurgists,
chemical engineers, corrosion engineers, etc. FactSage is widely known for its
ability to simulate complex chemical equilibria and process simulation, also
used in this study (Islam,
2015).
The simulation of chemical equilibria in
this study will be done in the 'Equilib' module in FactSage. The module's
function is Gibbs energy minimization, where the concentrations of chemical
species will be calculated when specified elements or compounds react or
partially react in a state of chemical equilibrium (Islam,
2015). The
reactants input, following the information from raw material characterization,
is presented in Table 4. For simplicity, volatile matter in the reductant is
assumed as methane, CH4, and the moisture is H2O.
The process will be conducted at 1300 to 1700°C and 1 atm. The database
selected for this simulation is FactPS for a pure substance, FToxid for oxides,
and FTstel for iron-bearing metal.
Figure
4 Nickel recovery with a different reductant
Figure 5 Liquid
metal mass-produced with a different reductant
Figure 6 Iron and
nickel concentrations in liquid metal with different reductant
The simulation of the blast furnace process of nickel laterite using anthracite coal and coconut shell charcoal as reductant, lime, SAAand the air is successfully done with FacSage software version 7.2, using FactPS, FToxid, and FSstel databases. The result of this simulation is often perceived as the ideal condition that cannot be achieved in real experimental work. However, the simulation gives us an insight of the upcoming further research results. In addition, software simulation can also save time, energy, and cost of the real experiment. The simulation shows that anthracite coal consistently gives AA results than coconut shell charcoal in liquid metal mass-produced and iron concentration. However, coconut shell charcoal may have the potential to substitute the conventional coal used in the blast furnace processes because the result is similar to anthracite coal. In addition, it can still yield nickel concentration above 2% and iron concentration above 96% at a temperature higher than 1400°C. Based on this simulation result, further experimental work can be targeted at temperatures above 1400°C.
The
authors are thankful for the aid from BRIN for the sample supply and the
Swinburne University Postgraduate Research Awards (SUPRAs), allowing access to
FactSage version 7.2 software.
Campos, A.M.A., Assis, P.S., 2021. Analysis of the influence of
biomass addition in coal mixture for metallurgical coke production. Global
Journal of Researches in Engineering, Volume 21(2), pp. 1–9
Chakraborty, S., 2014. Ferrous metallurgical process industry:
visakhapatnam steel plant–from conceptualization to commissioning. Treatise
on Process Metallurgy, Volume 3, pp. 1341–1374. Elsevier Ltd.
Chen, G.J., Hwang, W.S., Liu, S.H., Shiau, J.S., 2015. The effect
of bio-coal on the on the carbothermic reduction of laterite ores. Materials
Transactions, Volume 56(4), pp. 550–55
Chen, H., Morita, K., Ma, X., Chen, Z., Wang, Y., 2019. Boron removal
for solar-grade silicon production by metallurgical route: a review. Solar
Energy Materials and Solar Cells, Volume 203, p. 110169
Citrawati, F., Dwiwandono, R., Firmansyah, L., 2020. The effect of
ni on the formation of bainite in Fe-Ni lateritic steels through
semi-continuous cooling method. International Journal of Technology, Volume
11(1), pp. 60–70
Islam, M.S. 2015. Electrically enhanced slag-metal reactions.
Swinburne University of Technology
Kim, J., Dodbiba, G., Tanno, H., Okaya, K., Matsuo, S., Fujita, T.,
2010. Calcination of low-grade laterite for concentration of Ni by magnetic
separation. Minerals Engineering, Volume 23(4), pp. 282–288
Li, B., Wang, H., Wei, Y., 2011. The reduction of nickel from
low-grade nickel laterite ore using a solid-state deoxidisation method. Minerals
Engineering, Volume 24(14), pp. 1556–1562
Minister of Energy and Mineral Resources
Republic of Indonesia, 2013. Regulation of the minister of energy and mineral resources Republic
of Indonesia No. 20. Indonesia.
Petrus, H.T.B.M., Diga, A., Rhamdani, A.R., Warmada, I.W.,
Yuliansyah, A.T., Perdana, I., 2017. Lamtoro charcoal (l. Leucocephala) as bioreductor
in nickel laterite reduction: performance and kinetics study. In Journal of
Physics: Conference Series, Volume 817, p. 012065
Petrus, H.T.B.M., Putera, A.D.P., Sugiarto, E., Perdana, I.,
Warmada, I.W., Nurjaman, F., Astuti, W., Mursito, A.T., 2019. Kinetics on roasting
reduction of limonitic laterite ore using coconut-charcoal and anthracite
reductants. Minerals Engineering, Volume 132(2), pp. 126–133
Petrus, H.T.B.M., Putera, A.D.P., Warmada, I.W., Nurjaman, F.,
Astuti, W., Prasetya, A.,2022. Investigation on saprolitic laterite ore
reduction process using palm kernel shell charcoal: kinetics and phase
transformation. International Journal of Technology, Volume 13, pp. 565–574
Purnomo, C.W., Kesuma, E., Wirawan, S.K., Hinode, H., 2017. The development
of Lithium Ion recovery method by activated carbon and natural zeolite-based
adsorbent. ASEAN Journal of Chemical Engineering,
Volume 17(1), pp. 91–98
Putera, A.D.P., Sugiarto, E., Warmada, I.W., Petrus,
H.T.B.M., 2017. Coconut shell charcoal as a bioreductor in roasting
process of nickel laterite from pomalaa, southeast sulawesi: performance and
kinetics study. Materials Science Forum, Volume 901, pp. 182–189
Rao, M., Li, G., Jiang, T.A.O., Luo, J.U.N., Zhang, Y., Fan, X.,
2013. carbothermic reduction of nickeliferous laterite ores for nickel pig iron
production in china: a review. The Minerals, Metals & Materials Society,
Volume 65(11), pp. 1573–1583
Rasyid, M.H.A., Petrus, H.T., 2016. Study on biomass performance in
reduction of nickel laterite from Pomalaa, Sulawesi Tenggara. In AIP
Conference Proceedings, Volume 1755(1), p. 050007
Wanta, K.C., Astuti, W., Petrus, H.T.B.M., Perdana, I., 2022.
Product diffusion-controlled leaching of nickel laterite using low
concentration citric acid leachant at atmospheric condition. International
Journal of Technology, Volume 13(2), pp. 410–421
Yunus, N.A., Ani, M.H., Salleh, H.M., Rashid, R.Z., Akiyama, T.,
Purwanto, H., Othman, N.E., 2014. Effect of reduction roasting by using
bio-char derived from empty fruit bunch on the magnetic properties of malaysian
iron ore. International Journal of Minerals, Metallurgy and Materials,
Volume 21(4), pp. 326–330
Zhou, S., Wei, Y., Li, B., Wang, H., Ma, B., Wang, C., Luo,
X., 2017. Mineralogical characterization and design of a treatment process
for yunnan nickel laterite ore, China. International Journal of Mineral
Processing, Volume 159, pp. 51–59
