Enhancing the Quality of Cassava Starch-Based Wood Bioadhesive using Polyvinyl Alcohol
Published at : 24 Dec 2024
Volume : IJtech
Vol 15, No 6 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i6.5789
Budhijanto, Pancasakti, B.P., Tri Hartanto, D., 2022. Enhancing the Quality of Cassava Starch-Based Wood Bioadhesive using Polyvinyl Alcohol. International Journal of Technology. Volume 15(6), pp. 1886-1897
| Budhijanto | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No. 2, 55281, Yogyakarta, Indonesia |
| Bima Prasetya Pancasakti | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No. 2, 55281, Yogyakarta, Indonesia |
| Danang Tri Hartanto | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No. 2, 55281, Yogyakarta, Indonesia |
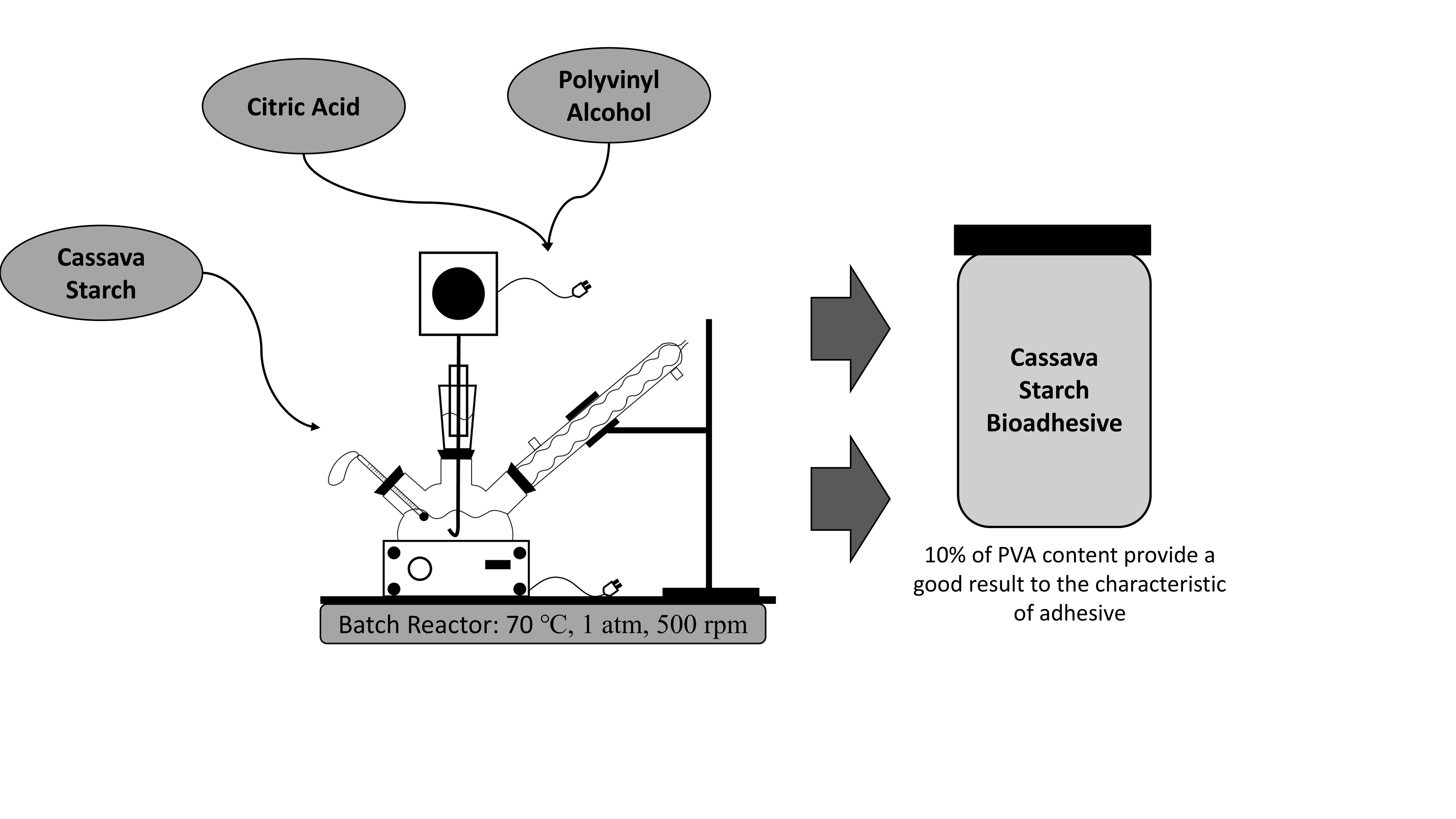
Many studies tried to innovate wood bioadhesives using renewable resources. This research attempted to synthesize wood
bioadhesive using cassava starch and polyvinyl alcohol (PVA). As a new
cross-linking agent, citric acid was added to the bioadhesive to ensure the bonding between cassava starch and PVA. PVA and
citric acid contain hydroxyl groups that can initiate polymerization with
starch. The
synthesis of bioadhesive has three steps, namely, acid hydrolysis, oxidation, and cross-linking reactions. The operating
condition was set at 70°C under atmospheric pressure for 90 minutes. The amount
of added PVA
varied at 0%, 10%, 20%, 30%, and 40% relative to the
weight of cassava starch. Physical properties such as viscosity, solid content, shelf life, and dry
and wet shear strength were analyzed to determine the best
composition of starch and PVA. Results showed that the addition of PVA could enhance the bioadhesive's
properties, especially its dry and wet shear strength.
Cassava starch; Citric acid; Polymerization; Polyvinyl alcohol; Wood bioadhesive
Wood adhesive is one of the essential materials in the wood industry (Dunky, 2017; Kaboorani and Riedl, 2011; Frihart, 2011) because of
its easy and fast application in joining a pair of wood pieces. Synthetic
adhesives such as phenol-formaldehyde and urea-formaldehyde are the popular types of adhesives
(?ehi?, Tali?, and Omer, 2023; Gonçalves
et al., 2021; Pizzi and Mittal, 2003). The industry has become comfortable with using this adhesive during
manufacturing because of its good performance in
gluing a couple of wood parts. However, this type of adhesive has a dangerous
influence on human health.
Synthetic adhesives contain highly
volatile compounds that could cause irritation and
respiratory problems in humans after prolonged contact (David and
Niculescu, 2021; Kumar
and Pizzi, 2019; May, 2017).
Bioadhesive is an alternative to synthetic adhesives and has sufficient capability to glue a pair of wood pieces. Moreover, it offers several advantages, such as renewability, low cost, easy processing, reusability, and environmental friendliness (Borges et al., 2023; Balgude, Sabnis, and Ghosh, 2017; Raydan et al., 2021). However, this adhesive has weak strength and lousy durability compared with synthetic adhesives. Many researchers have tried to innovate and develop bioadhesives from many renewable resources to obtain excellent adhesive characteristics. Cassava starch is an abundant resource that shows potential as the raw material for adhesives (Admase, Mersha, and Kebede, 2024; Yu et al., 2016; Ozemoya, Ajisegiri, and Idah, 2007). Due to its easy gelatinization and modification suitable for developing new wood bioadhesives (Wang et al., 2017; Zhang et al., 2015; Wang et al., 2012). Many studies were conducted to improve the quality of cassava starch as a bioadhesive. Modifications, such as hydrolysis, oxidation, and gelatinization, were carried out to obtain high-quality starch bioadhesives. However, starch's retrogradation limits its performance as a bioadhesive. Several studies attempted to add an acid catalyst (Gadhave et al., 2020; Monroy, Rivero, and García, 2019) or a surfactant (Li et al., 2014) to prevent this retrogradation.
Starch retrogradation can be reduced by
binding the starch with other monomers or polymers to
form a new polymer model. Polyvinyl alcohol (PVA) is an example of a
polymer that can be added to
starch to improve the 'latter's adhesive properties (Elgharbawy et al., 2024;
Farshi et al., 2024; Jaffe
and Rosenblum, 1990).
PVA is a synthetic polymer that is cheap,
non-toxic, and has high water solubility and elasticity (Chousidis, 2024; Barleany et
al., 2020; Gaaz et al., 2015). PVA can be applied as a nanofiber membrane. The
addition of 5% ramie cellulose nanocrystal (CNC) as
reinforcement can increase the tensile strength by 2 times more than before it was added (Xu et al., 2024; Nguyen et
al., 2023; Rochardjo et
al., 2021). Another study stated that
adding a copolymer in the form of PVA and 2-ethylhexyl acrylate (2-EHA) grafted
with ammonium persulfate (APS) in mortar could increase physical properties
such as compressive strength and reduce water absorption (Bekbayeva
et al., 2020). The hydroxyl groups of PVA provide an opportunity to form a new polymer between PVA and starch (Sridach, Jonjankiat,
and Wittaya, 2013; Reddy
and Yang, 2010). As a new natural cross-linking agent, citric acid can initiate polymerization to ensure
PVA–starch bonding. In this research, we
tried to synthesize a new bioadhesive
polymer from cassava starch and PVA using citric acid as the cross-linking
agent. Physical properties such as
viscosity, durability, and shear strength were analyzed to evaluate the adhesive quality of the
bioadhesive polymer.
2.1. Materials
The
following are the
materials used in this research: cassava starch (PT. Budi Acid Jaya, Tbk), ammonium persulfate ((NH4)2S2O8)
(LPPT UGM, Indonesia), polyvinyl alcohol (PVA) (99% hydrolyzed, Mw 89,000 – 99,800, Merck KgaA Company, Germany), sodium dodecyl sulfate (SDS)
(CV. Gudang Kimia, Indonesia), 37%
hydrochloric acid (HCl) (Merck
KgaA Company, Germany), and wooden board (Tectona grandis) (UD. Makmur Jaya, Indonesia).
2.2. Synthesis of Bioadhesive
In
this experiment, approximately 50 g of cassava starch was dissolved in 100 mL
of 0.5 M HCl. The resulting solution was placed in a three-neck flask and
heated to 70°C with stirring at 500 rpm using a mercury stirrer. After 10
minutes, 0.4 g of (NH4)2S2O8 was
added to the solution, followed by the addition of 1 g of SDS after 30 minutes.
Separately, 5 g of PVA was dissolved in 50 mL of Aquadest and added to the
three-neck flask. Then, 0.4 g of citric acid was added to the solution and
stirred for 1 hour. The experiment was repeated with different amounts of PVA
(0, 10, 15, and 20 g) using the same procedure.
Figure 1 The Illustration of Bioadhesive Synthesis in the Temperature of 70 ?
and Pressure 1 atm
2.3. Viscosity Analysis
The
'adhesive's viscosity was evaluated in accordance
with ASTM D1084. The
adhesive was transferred to a beaker glass, and a suitable spindle was installed by considering the thickness of the adhesive. The size and
rotating speed (rpm) of the spindle in the DV-E Brookfield Viscometer were controlled. Viscosity was recorded as
the value consistently shown on the monitor. The allowed error for viscosity was in the
range of 10%–90%.
2.4. Shelf-Life
Analysis
The
shelf life of the adhesive was measured in accordance
with ASTM D1337. The
sample was kept at room temperature with 50% relative humidity, and its viscosity was tested every 7 days for 31 days. The thickness was evaluated
in accordance
with ASTM D1084.
2.5. Solid Content Analysis
The solid content
of the adhesive was evaluated in accordance with ASTM-D2369. In brief, 1 g of the adhesive was placed in a petri dish and dried in the oven for 60
minutes at 110 ± 2.5°C. The sample was then cooled and stabilized in a desiccator for
10 minutes and finally weighed. The solid
content of the adhesive was calculated using Equation 1:
where
S is solid content (%), m0 is the weight of the petri dish (g),
m1 is the weight of the petri dish and sample before heating (g),
and m2 is the weight of the petri dish and sample after heating (g).
The analysis was repeated three times, and the results were averaged.
2.6. Dry Shear Strength Analysis
Dry
shear strength was evaluated in accordance with ASTM D906. Wood pieces with 30 × 25 × 2 mm dimensions were glued using the adhesive and pressed under 3.27 × 10-2
N/mm2 for 24 hours. The wood samples were stored at room temperature with 50% RH for 7 days, and their maximum load was measured using a universal testing machine at 2 mm/min of testing speed. The shear
strength was calculated using Equation 2:
2.7. Wet Shear Strength Analysis
Wet shear strength was evaluated in accordance
with ASTM D1183. Dry shear strength was measured by determining the exact dimension of the wood that was glued and pressed under 3.27 × 10-2
N/mm2. After 24 hours, the sample was immersed in water for another
24 hours. The sample was dried for 24 hours and stored at 50% RH and at room
temperature for 7 days. Afterward, the maximum load of the samples was measured
using a universal testing machine at 2 mm/min of testing speed. The wet shear strength was calculated
using Equation 2. The measurement was repeated five times, and the obtained values were averaged.
2.8. Fourier Transform Infrared (FTIR)
Spectroscopy
FTIR spectroscopy was applied to identify the functional groups in the
samples using spectrophotometer
FT-IR Mb 3000 (LPPT UGM, Yogyakarta, Indonesia). The spectra ranged between 650 and 4000 cm-1, and 32
scans were performed.
2.9. DinoLite
Microscopy and Scanning Electron Microscopy (SEM)
The surfaces and morphology of the bioadhesive were analyzed using a DinoLite microscope and a SEM from LPPT UGM, Yogyakarta, Indonesia.
Various PVA and starch compositions were examined to determine the best combination that would provide
the best performance for the bioadhesive. The PVA: starch ratios of 1:10, 2:10, 3:10, 4:10, and 0:10 (wt./wt.) were labeled as SP1, SP2, SP3, SP4, and SP0, respectively (the blank
sample: regular bioadhesive without PVA). The
experiment was carried out using a batch reactor, which is easy to control and can provide the best conversion in the
reaction.
3.1. Reaction
Mechanisms of Adhesive Synthesis
Several mechanisms are involved in bioadhesive synthesis. The first process involved hydrolysis and gelatinization using HCl solution as the catalyst at 70°C under atmospheric pressure. In this step, the glycosidic bonds in cassava starch were destroyed, resulting in acid-thinned starch (ATOS). The starch's primary
alcohol or primary hydroxyl groups were then oxidized using (NH4)2S2O8 (Imanzadeh
et al., 2007).
This
process aimed to form the carboxyl
groups (-COOH) in the structure to support polymerization. The other hydroxyl groups or secondary alcohol could be oxidized to carbonyl groups (-CO). SDS and PVA were
dissolved in aquadest and added to the
mixture. SDS was added to prevent
retrogradation during cassava starch gelatinization. After 10 minutes, citric acid was added to the
mixture as the cross-linking
agent. The two possible reaction paths in cross-linking reactions are
polymerization between starch molecules and polymerization between starch and PVA. These synthesis processes are shown in Figure 3.
This solution
polymerization reaction requires a non-monomeric solvent, aquadest (H2O) (Ahmed
and Rashid, 2022). PVA is dissolved in aquadest (liquid phase),
so the opportunity to in-contact with oxidized starch, PVA, and citric acid is
greater. Moreover, the heat of the polymerization reaction can be
removed by water. The hydroxyl group of PVA interacts with the carboxylic group
of citric acid, thus forming an ester. The molecules formed become larger, so
the average molecular weight increases.
Figure 3 Sequence of reaction in starch/polyvinyl alcohol adhesive formation
with citric acid addition
3.2. Viscosity of Adhesives
Viscosity was analyzed following ASTM D1084 to measure the thickness of the
adhesive using a spindle with the lowest rotating
speed. This setting was selected because the adhesive is a
non-Newtonian thixotropic fluid; hence, its viscosity will decrease over time (Bird, Stewart, and Lightfoot, 1960). The viscosity was calculated from the average of
measured viscosities on the Brookfield monitor.
The viscosity of adhesives is shown in Figure 4. First, the viscosity decreased from 2118 cP to
106.2 cP and then gradually increased to 984, 2312, and 5127 cP. When the PVA
content was 10%, the viscosity
decreased due to the excess water in SP1. Meanwhile, the viscosity increased with PVA content due to the increase in the average molecular
weight caused by the cross-linking
reaction. PVA also introduced some solid particles into
the adhesive, thus increasing its viscosity.
Figure 4 Viscosity of the adhesives with various
polyvinyl alcohol additions
Viscosity is related to the ability of the adhesive to cover the
surface of the wood easily.
Therefore, adhesives with high viscosity are not desirable. SP1 had the lowest value of
viscosity. The addition of water and PVA in this sample rendered it easy to apply. However, this addition affected the composition of solids and liquids in the
adhesive. The polymerization between starch or
starch/PVA also produced a large amount of water. This reaction involved condensation because of the interactions between the hydroxyl group on
PVA and carboxylic acid on citric acid and between the hydroxyl group on starch and carboxylic acid on citric acid (Figure 3).
3.3. Shelf Life of Adhesives
The shelf life of the adhesive was evaluated
to estimate its durability. The
samples' viscosity was measured per ASTM D1084 every week for 31 days. The
results are shown in Figure 5.
The viscosity of each sample increased over
time. After 24 days, SP4 substantially thickened, so its viscosity could not be measured using the Brookfield Viscometer. The viscosity
increased due to the contact between the adhesive and air trapped in the
container. An adhesive with high viscosity
is difficult to apply to any wood 'specimen's
surface.
Figure 5 Viscosity of bioadhesives in a certain period
Figure 5 Viscosity of bioadhesives in a certain period
The
significantly increased viscosity of SP4 shows that excessive PVA addition can
have a negative impact on the quality of the adhesive. Additionally, clumping
occurred in the adhesive that had been stored for a long period of time. This phenomenon increased the viscosity level of the adhesive
and decreased its adhesion strength, thus making it
nonreusable.
Solidification is the other reason for the increased viscosity of the adhesive. Excessive PVA addition will increase the number of
solid particles in the adhesive, allowing PVA and starch to solidify. This phenomenon is plausible because no other
additives have been incorporated into the adhesives.
3.4. Solid Content of Adhesives
The adhesives' solid content was measured per ASTM D2369 to identify their volatility level. High content of solid
particles will decrease the volatility of the adhesive.
The solid content of the adhesives
is shown in Figure 6. First, the value decreased from 30.62%
to 25.16% and then increased
gradually to 28.00%, 30.19%, and 32.70%. When the PVA content was 10%, the solid content decreased due to the low amount of solid particles in
SP1. The solid content increased with the amount of PVA addition because many nonvolatile molecules were formed after the cross-linking reaction.
Figure 6 Solid content of the adhesives with various polyvinyl alcohol additions
Compared with SP0, the other samples had lower solid content, except for SP4. The
reaction between PVA and starch increased the water content of the samples. Meanwhile, PVA addition also increased water accumulation in the adhesive. The
excess water will reduce the solid content of the adhesive because the water will evaporate at
oven temperature.
3.5. Dry Shear Strength of Adhesives
Shear strength is an essential parameter
because it estimates the bonding strength of the adhesive. The shear strength
measured in the dry condition is shown in Figure 7. The dry shear strength
increased with the amount of PVA addition. In the dry state, the shear strength increased from 0.55 MPa to 2.54
MPa. Compared with SP0, SP4 had
increased initial shear strength by 316.82% in the dry state and 678.13% in the wet
state. These results
indicated that the addition of PVA increases the shear strength of the adhesives.
The cross-linking reaction between starch and PVA enhances the starch-based
adhesive's bonding strength.
PVA addition can improve the chemical structure of
the cassava
starch adhesive. The bond of cassava starch–citric acid–cassava starch or
cassava starch–citric acid–PVA rendered the resulting polymer highly
stable. The interaction between hydroxyl groups (-OH) and carboxyl groups
(-COOH) formed crosslinked
molecules. The amount of these groups decreased, and the adhesive shear strength increased due to the absence of affinity effects on the water in the air.
Figure 7 Dry shear strength of the adhesives with various polyvinyl alcohol additions
3.6. Wet Shear Strength of Adhesives
Wet shear strength was analyzed per ASTM D1183 to determine theadhesive's resistance under moist or wet conditions. The adhesive's strength under various conditions must be evaluated to determine whether it can be used in the wet state. The results of the wet
shear strength analysis are shown in Figure 8.
Figure 8 Wet shear strength of the adhesives with various polyvinyl alcohol additions
The wet shear strength increased with PVA addition. The shear strength
increased from 0.32 MPa to 2.49 MPa. All adhesives had lower shear strength in the wet state than in the dry
state. The shear strength
decreased due to the high affinity between water and cassava starch. The
hydroxyl groups (-OH) in cassava starch have an affinity for water, allowing cassava starch to be used as an adhesive that can dissolve in water. The dissolution of the adhesive into the water will
affect the adhesive used during the gluing process. When a large
amount of adhesive dissolves,
the adhesion strength becomes weak. This affinity to water is
caused by the similarity of
polarity between the hydroxyl groups (-OH) and water. In some samples, the
added PVA can prevent the cassava starch adhesive from dissolving during
soaking. The PVA addition produced
ester bioadhesives (crosslinked starch) and increased the water accumulation in the adhesive. The high-water content in the adhesive reduced the water resistance and broke the bonding of the wood specimens. Thus, the solubility of the adhesive increased, leading to a low
bonding strength under moist conditions.
Compared with the dry shear strength, the wet shear strength decreased significantly.
Table 1 shows the decrease in adhesive shear strength in each sample. In particular, SP4 experienced a reasonably small decrease
in shear strength. Therefore, PVA addition can reduce the solubility of cassava starch in
water. This effect can be attributed to the reduction in the hydroxyl groups (-OH) because of polymerization. Reducing the number of hydroxyl groups will reduce the solubility and increase the water resistance of the adhesive.
Table 1 Reduction in the shear strength of the adhesives after being immersed in water
3.7. FTIR of Adhesives
FTIR spectroscopy was carried out to observe
the structural changes of the starch-based adhesives during the reaction. The
molecular structures were compared among cassava starch, ATOS, SP0, and SP3. The SP4 was not used because it had dried out. The analysis result is
shown in Figure 2. At wavenumber 1022.43 cm-1, the peak intensity of C–O bonds decreases from cassava starch to ATOS. Meanwhile, the peak intensity of O–H groups increases at
wavenumber 3330.34 cm-1. This finding indicated the occurrence of hydrolysis that destroyed the C-O bonds in amylose and formed the hydrolyzed starch. In addition, O-H bonds in cassava starch increase because hydroxyl groups are formed more
in each single molecular hydrolyzed starch.
SP0 has a higher peak intensity at 1022.43 cm-1 and 1636.4 cm-1.
This is due to the addition of citric acid to SP0 from oxidized starch. The
number of carboxyl groups increases and ester groups may start to form. Overall, no significant change was observed between SP0 and SP3 due to the compensatory role of the new O-H bonds generated from the polymerization between starch/PVA and citric acid. However, SP3 has a broader peak than SP0 in the
3000-3500 cm-1. The hydroxyl group in PVA reacts with a carboxylic
group from citric acid, so it was resulting in shifts, changes, and broadening
of the absorption band SP3 to form ester groups
3.8. Morphology of
Adhesive
The morphology of adhesives was analyzed using a DinoLite microscope and a SEM. The bioadhesive films with citric acid
addition (Figure 9b and 10b) were
smoother than those of the adhesive without the cross-linking agent (Figure 9a and 10a). This finding
indicated that citric acid
provides good homogeneity to the adhesive solution and increases the capability of the adhesive to bond the wood pieces and create a strong bonding.
Figure 10 Morphology of the adhesive without citric acid addition (a) and with citric acid addition
(b) visualized using scanning
electron micrographs
PVA addition improves the properties of cassava starch-based bioadhesive. The viscosity and solid content of bioadhesives tend to increase with the PVA content. The dry and wet shear strength are enhanced when the PVA amount is increased. The dry and wet shear strengths of SP4 are 316.82% and 678.13% higher than those of the adhesive without PVA, respectively. However, an excessive amount of PVA addition could drastically increase the viscosity of the adhesive and accelerate the thickening process, leading to the poor durability and reusability of the bioadhesive. Therefore, the suitable composition of PVA addition to the starch adhesive was in sample S1 (10% of PVA content) because it offered a low viscosity value. On the other hand, this sample had enough value of shear strength to apply as wood bioadhesive directly. For future research, it is crucial to analyze the characteristics of adhesive toxicity and vary the use of starch as raw material.
This research was supported by Faculty Research Grants 2021 No: 14543/UN1.FTK/SK/HK/2021 from Faculty of Engineering, Universitas Gadjah Mada.
Admase, A.T., Mersha, D.A.,
Kebede, A.Y., 2024. Cassava Starch-Based Hot Melt Adhesive for Textile
Industries. Scientific Reports, Volume 14, p. 20927, https://doi.org/10.1038/s41598-024-70268-y
Ahmed, B.Y.,
Rashid, S.O., 2022. Synthesis, Characterization, and Application of Metal-Free
Sulfonamide-Vitamin C Adduct to Improve the Optical Properties of PVA Polymer. Arabian
Journal of Chemistry, Volume 15(10), p. 104096, https://doi.org/10.1016/j.arabjc.2022.104096
Balgude, D.,
Sabnis, A., Ghosh, S.K., 2017. Synthesis and Characterization of Cardanol Based
Reactive Polyamide for Epoxy Coating Application. Progress in Organic
Coatings, Volume 104, pp. 250–262., https://doi.org/10.1016/j.porgcoat.2016.11.012
Barleany, D.R., Ananta, C.V., Maulina, F., Rochmat, A., Alwan, H., Erizal, 2020. Controlled Release of Metformin Hydrogen Chloride From Stimuli-Responsive Hydrogel Based On Poly(N-Isopropylacrylamide)/Chitosan/Polyvinyl Alcohol Composite. International Journal of Technology, Volume 11(3), pp. 511–521, https://doi.org/10.14716/ijtech.v11i3.233014716/ijtech.v11i3.2330
Bekbayeva, L., Negim, E.,
Gulzhakhan, Y., Ganjian, E., 2020. Utilization of Poly(Polyvinyl
Alcohol-g-2-Ethylhexyl Acrylate) as Admixture for Mortar. International
Journal of Technology, Volume 11(2), pp. 259–268, https://doi.org/10.14716/ijtech.v11i2.3927
Bird, R.B., Stewart, W.E.,
Lightfoot, E.N., 2002. Phenomena, Transport. Transport Phenomena, Wiley and
Sons, pp. 582–605, ISBN 0-471-41077-2
Borges, C.S.P., Jalali, S.,
Tsokanas, P., Marques, E.A.S., Carbas, R.J.C., da Silva, L.F.M., 2023.
Sustainable Development Approaches through Wooden Adhesive Joints Design. Polymers,
Volume 15(1), p. 89, https://doi.org/10.3390/polym15010089
?ehi?, M., Tali?, H., Omer,
S.E., 2023. Eco-Friendly Adhesives for Wood-Based Composite Production. IOP
Conference Series: Materials Science and Engineering, Volume 1298, p.
012013, https://doi.org/10.1088/1757-899X/1298/1/012013
Chousidis, N., 2024.
Polyvinyl Alcohol (PVA)-Based Films: Insights from Crosslinking and Plasticizer
Incorporation. Engineering Research Express, Volume 6, p. 025010, https://doi.org/10.1088/2631-8695/ad4cb4
David, E., Niculescu, V.C.,
2021. Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence
and Mitigation Using Nanomaterials. International Journal of Environmental
Research and Public Health, Volume 18(24), p. 13147, https://doi.org/10.3390/ijerph182413147
Dunky, M., 2017. Adhesives
in the Wood Industry. In: Pizzi, A., Mittal, K.L. (Eds.), Handbook of
Adhesive Technology, 3rd Edition. Taylor and Francis, https://doi.org/10.1201/9781315120942
Elgharbawy, A.S., El
Demerdash, A.G.M., Sadik, W.A., Kasaby, M.A., Lotfy, A.H., Osman, A.I., 2024.
Synthetic Degradable Polyvinyl Alcohol Polymer and Its Blends with Starch and
Cellulose—A Comprehensive Overview. Polymers, Volume 16(10), p. 1356, https://doi.org/10.3390/polym16101356
Farshi, P., Mirmohammadali,
S.N., Rajpurohit, B., Smith, J.S., Li, Y., 2024. Pea Protein and Starch:
Functional Properties and Applications in Edible Films. Journal of
Agriculture and Food Research, Volume 15, p. 100927, https://doi.org/10.1016/j.jafr.2023.100927
Frihart, C.R., 2011. Wood
Adhesives: Vital for Producing Most Wood Products. Forest Products Journal,
Volume 61(1), pp. 4–12, https://doi.org/10.13073/0015-7473-61.1.4
Gaaz, T.S., Sulong, A.B.,
Akhtar, M.N., Kadhum, A.A.H., Mohamad, A.B., Al-Amiery, A.A., 2015. Properties
and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their
Nanocomposites. Molecules, Volume 20(12), pp. 22833–22847, https://doi.org/10.3390/molecules201219884
Gonçalves, D., Bordado,
J.M., Marques, A.C., dos Santos, R.G., 2021. Non-Formaldehyde, Bio-Based
Adhesives for Use in Wood-Based Panel Manufacturing Industry—A Review.
Polymers, Volume 13(23), p. 4086, https://doi.org/10.3390/polym13234086
Imanzadeh, G.H., Zamanloo,
M.R., Mansoori, Y., Khodayari, A., 2007. Aqueous Media Oxidation of Alcohols
with Ammonium Persulfate. Chinese Journal of Chemistry, Volume 25(6), pp.
836–838, https://doi.org/10.1002/cjoc.200790153
Jaffe, L., Rosenblum, M.,
1990. Poly (Vinyl Alcohol) for Adhesives. Handbook of Adhesives, pp. 401–407, https://doi.org/10.1007/978-1-4613-0671-9_22
Kaboorani, A., Riedl, B.,
2011. Effects of Adding Nano-Clay on Performance of Polyvinyl Acetate (PVA) as
a Wood Adhesive. Composites Part A: Applied Science and Manufacturing,
Volume 42(8), pp. 1031–1039, https://doi.org/10.1016/j.compositesa.2011.04.007
Kumar, R.N., Pizzi, A.,
2019. Adhesives for Wood and Lignocellulosic Materials. In: Adhesion and
Adhesives: Fundamental and Applied Aspects. Wiley-Scrivener, https://doi.org/10.1002/9781119605584
Li, Z., Wang, J., Cheng,
L., Gu, Z., Hong, Y., Kowalczyk, A., 2014. Improving the Performance of
Starch-Based Wood Adhesive by Using Sodium Dodecyl Sulfate. Carbohydrate
Polymers, Volume 99, pp. 579–583, https://doi.org/10.1016/j.carbpol.2013.08.062
?ebkowska, M.,
Za??ska-Radziwi??, M., Tabernacka, A., 2017. Adhesives Based on Formaldehyde –
Environmental Problems. BioTechnologia, Volume 1(1), pp. 53–65, https://doi.org/10.5114/bta.2017.66617
Monroy, Y., Rivero, S., García, M.A., 2019. Sustainable Panels
Design Based on Modified Cassava Starch Bioadhesives and Wood Processing
Byproducts. Industrial Crops and Products, Volume 137(2), pp. 171–179, https://doi.org/10.1016/j.indcrop.2019.04.062
Nguyen, Y.T.H., Ly, T.B.,
Bui, B.A.T., Le, P.K., 2023. Biodegradable Polyvinyl Alcohol Composite Film
Reinforced by Crystalline Nanocellulose from Rice Straw. Chemical
Engineering Transactions, Volume 106, p. 83, https://doi.org/10.3303/CET23106083
Ozemoya, P.O., Ajisegiri,
E., Idah, P.A., 2007. Production of Adhesives from Cassava Starch. Leonardo
Electronic Journal of Practices and Technologies, Volume 10 (1), pp. 93–98,
https://doi.org/10.1088/1742-6596/1378/3/032079
Pizzi, A., Mittal, K.L.,
2003. Urea-Formaldehyde Adhesives. In: Handbook of Adhesive Technology, 2nd
Edition. Marcel Dekker, Inc., https://doi.org/10.1201/9780203912225
Raydan, N.D.V., Leroyer,
L., Charrier, B., Robles, E., 2021. Recent Advances on the Development of
Protein-Based Adhesives for Wood Composite Materials—A Review. Molecules,
Volume 26(24), p. 7617, https://doi.org/10.3390/molecules26247617
Reddy, N., Yang, Y., 2010.
Citric Acid Cross-Linking of Starch Films. Food Chemistry, Volume 118(3), pp.
702–711, https://doi.org/10.1016/j.foodchem.2009.05.050
Rochardjo, H.S.,
Fatkhurrohman, Kusumaatmaja, A., Yudhanto, F., 2021. Fabrication of
Nanofiltration Membrane Based on Polyvinyl Alcohol Nanofibers Reinforced with
Cellulose Nanocrystal Using Electrospinning Techniques. International
Journal of Technology, Volume 12(2), pp. 329–338, https://doi.org/10.14716/ijtech.v12i2.4173
Sridach, W., Jonjankiat,
S., Wittaya, T., 2013. Effect of Citric Acid, PVOH, and Starch Ratio on the
Properties of Cross-Linked Poly(Vinyl Alcohol)/Starch Adhesives. Journal of
Adhesion Science and Technology, Volume 27(15), pp. 1727–1738, https://doi.org/10.1080/01694243.2012.753394
Wang, Y., Xiong, H., Wang,
Z., Zia-ud-Din, Chen, L., 2017. Effects of Different Durations of Acid
Hydrolysis on the Properties of Starch-Based Wood Adhesive. International
Journal of Biological Macromolecules, Volume 103, pp. 819–828, https://doi.org/10.1016/j.ijbiomac.2017.05.102
Wang, Z., Li, Z., Gu, Z.,
Hong, Y., Cheng, L., 2012. Preparation, Characterization and Properties of
Starch-Based Wood Adhesive. Carbohydrate Polymers, Volume 88(2), pp. 699–706, https://doi.org/10.1016/j.carbpol.2012.01.023
Xu, Y., Wu, Z., Li, A.,
Chen, N., Rao, J., Zeng, Q., 2024. Nanocellulose Composite Films in Food
Packaging Materials: A Review. Polymers, Volume 16(3), p. 423, https://doi.org/10.3390/polym16030423
Yu, H., Fang, Q., Cao, Y.,
Liu, Z., 2016. Effect of HCl on Starch Structure and Properties of Starch-Based
Wood Adhesives. BioResources, Volume 11(1), pp. 1721–1728, https://10.15376/biores.11.1.1721-1728
Zhang, Y., Ding, L., Gu,
J., Tan, H., Zhu, L., 2015. Preparation and Properties of a Starch-Based Wood
Adhesive with High Bonding Strength and Water Resistance. Carbohydrate
Polymers, Volume 115, pp. 32–37, https://doi.org/10.1016/j.carbpol.2014.08.063
