The Light Features and Bredigite Layout for Orthosilicate Phosphor in WLED Devices
Corresponding email: hodangsang@tdtu.edu.vn
Published at : 28 Jun 2023
Volume : IJtech
Vol 14, No 4 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i4.5785
Thi, M.H.N.,Thai, N.L., Bui, T.M., Ho, S.D., 2023. The Light Features and Bredigite Layout for Orthosilicate Phosphor in WLED Devices. International Journal of Technology. Volume 14(4), pp. 911-920
| My Hanh Nguyen Thi | Faculty of Mechanical Engineering, Industrial University of Ho Chi Minh City, Ho Chi Minh City, Vietnam |
| Nguyen Le Thai | Faculty of Engineering and Technology, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam |
| Thuc Minh Bui | Faculty of Electrical and Electronics Engineering, Nha Trang University, Nha Trang City, Vietnam |
| Sang Dang Ho | Faculty of Electrical and Electronics Engineering, Ton Duc Thang University, Ho Chi Minh City, Vietnam |
We created a green phosphor Ca14-xEuxMg2[SiO4]8
or CMS: Eu2+ to be utilized in WLED devices. The phosphor
offers a wide spectrum achieving the highest value of 505 nm when excited at
400 nm, as a result of a shift between the excited state of 4f 65d
and the ground state of 4f 7 in an ion of Eu2+.
The interactivity of dipole-dipole appeared to be a primary power shift for the
electrical multipolar nature of the phosphor. We acquired a critical distance
measured at 12.9as well as 14.9
via a critical Eu2+ concentration as well
as the Dexter hypothesis on power shift. Via an encapsulant, we combined CMS:Eu2+
as well as a phosphor in red with a LED device having a
value of 395 nm and managed to acquire white
illumination having a CRI value measured at 91 at a 20-milliampere forward bias
current. In addition, we also examined the layout as well as the light features
in CMS:Eu2+.
Color homogeneity; Double-layer phosphor; Luminous flux; Monte Carlo theory; WLEDs
Computational
Simulation
2.1. Preparation
of green-emitting Ca14-xEuxMg2[SiO4]8 (CMS:Eu2+) phosphor
Figure 1 Photograph of WLEDs
2.2. Spectra
optimization of the CCT
Figure 2 (a) Discharge and emission spectra in CMS:Eu2+ when excited
at 400 nm under different concentrations of Eu2+ (x). (b) The location for
the maximal discharge. (c) The relative intensity of discharge along with
replacement of Eu2+ (x)
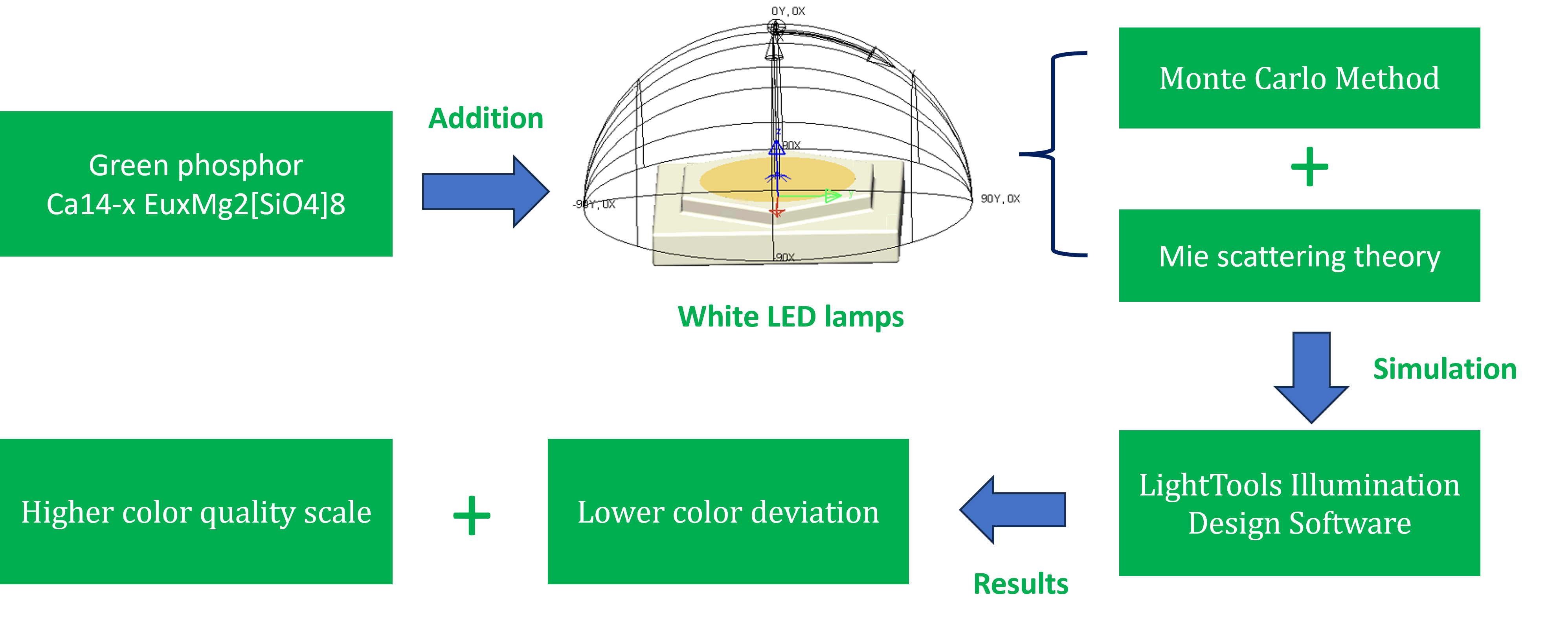
With
the use of the software LightTools 9.0 and the Monte Carlo approach, the flat
phosphor sheet for a multi-chip WLED (MCW-LED) was reproduced (Zhang et al.,
2021). The recreation procedure would have two main phases. First, assessing
as well as constructing the layout samples along with optical characteristics
in MCW-LED lights. Second, managing the phosphor mixture and the light
influences through many CMS:Eu2+
doping amount percentages. For the assessment of YAG:Ce3+ and CMS:Eu2+ compound’s
effects on WLED lights’ performance, generating variants would be compulsory.
Examining two kinds of compounds under the median CCT values of 3000 K, 4000 K,
and 5000 K, a remote phosphor layout with two sheets would be necessary. Via
Figure 1, the modeling illustration of the MCW-LED with a conformal phosphor
structure and a great 8500 K CCT value is presented. It also reveals that the
initial MCW-LEDs’ recreation does not involve CMS:Eu2+. The WLED components’ dimensions can be
listed as follows. A reflector with 2.07×8×9.85 mm for height and bottom- and
top-surface length, respectively; a 0.08 mm thick film of conformal phosphor
compounding would be daubed on the chips; a set of nine chips with a size of
1.14×0.15 mm (length × height) and radiant-flux value of 1.16 W, and 453 nm
peak wavelength for each is attached to LED reflector’s gap.
Figure 7 illustrates how the
concentration of green phosphor CMS:Eu2+
influence the emission power of the WLED at 4000 K and 8000 K, from which the producer can determine suitable CMS:Eu2+ concentration to
apply to their LED products. If they want to get high and strong luminescence
for backlight application, for example, they can allow a minor deduction in the
chromatic features. As depicted in both Figures 7(a) and 7(b), two critical
spectral regions for white light generation, 420-480 nm, and 500-640 nm are
improved with increasing CMS:Eu2+
concentration, suggesting the enhancement in the luminescent output of the WLED
model. Additionally, the addition of green phosphor
seems to stimulate the emission and dispersion of blue light, which will
contribute to increasing the possibility of blue-light conversion and
extraction. Thus, the active dispersion and color consistency could be
improved. This result is crucial and essential for the application of CMS:Eu2+ phosphor to serve
the enhancement of WLED-lighting performance, especially at high CCT like 8000
K.
Figure 11 CQS in the WLED devices with respective CMS:Eu2+ concentrations: (a) 3000 K-5000 K; (b) 6000
K-8000 K
Our study investigates
how the green phosphor CMS:Eu2+
would impact the lighting features in the two-sheet phosphor layout. Judging
the Monte Carlo recreations, this phosphor would be appropriate for the task of
augmenting hue homogeneity, which can be applied to WLED devices possessing hue
temperature measured at 5600 K and greater than 8500 K. Our study has managed
to augment the hue output as well as the lumen. Achieving these elements can be
fairly difficult in the case of remote phosphor layouts. On the other hand,
there is a small downside to CRI as well as CQS. If the CMS:Eu2+ concentration rises too high, both of these
elements would fall considerably. As such, choosing an appropriate
concentration by considering the producer’s requirements is very important. Our
study can be useful data for the task of acquiring superior hue homogeneity as
well as lumen in WLED devices.
This research is supported by Industrial
University of Ho Chi Minh City (IUH) under grant number 138/HD-DHCN.
Alexeev, A., Linnartz, J.M.G., Arulandu,
K., Deng, X., 2021. Characterization of Dynamic Distortion in LED Light Output
for Optical Wireless communications. Photonics Research, Volume 9(6), pp. 916–928
Bindai, S., Annapurna, K., Tarafder, A.,
2019. Realization of Phosphor-in-Glass Thin Film on Soda-Lime Silicate Glass with
Low Sintering Temperature for High Color Rendering White LEDs. Applied Optics,
Volume 58(9), pp. 2372–2381
Chang, Y., Shih, H., Liu, C., Chen, H.,
Tsai, S., Li, K., Han, P., Cheng, W., 2021. Laser-Assisted LED for Adaptive-Driving-Beam
Headlights Employing Ultra-Reliable Single Crystal Phosphor for Autonomous
Vehicles. Optics Express, Volume 29(17), pp. 26466–26473
Chen, Y., Wang, J., Zhang, J., Cao, G.,
2020. Light Source for Comfortable Lighting and Trapping Pests in Tea Gardens
Based on Solar-Like Lighting. Applied Optics, Volume 59(27), pp. 8459–8464
Hakim, F.N., Muhamadinah, Y.,
Atthaillah, A., Mangkuto, R.A., Sudarsono, A.S., 2021. Building Envelope Design
Optimization of a Hypothetical Classroom Considering Energy Consumption,
Daylighting, and Thermal Comfort: Case Study in Lhokseumawe, Indonesia. International
Journal of Technology, Volume 12(6), pp. 1217–1227
Huu, P.D., Thi, D.A.N., 2022. Selection of multi-layer
remote phosphor structure for heightened chromaticity and luminous performance
of white light-emitting diodes. International Journal of Technology,
Volume 13(4), pp. 837–847
Hsin, S., Hsu, C., Chen, N., Ye, C., Ji,
G., Huang, K., Hsieh, H., Wu, C., Dai, C., 2021. Design and Fabrication of A
Downlight Luminaire with a Dual Frusto-Conical Reflector. Applied Optics,
Volume 60(25), pp. 7775–7783
Jain, B., Velpula, R.T., Bui, H.Q.T.,
Nguyen, H.D., Lenka, T.R., Nguyen, T.K., Nguyen, H.P.T., 2020. High Performance
Electron Blocking Layer-Free Ingan/Gan Nanowire White-Light-Emitting Diodes. Optics
Express, Volume 28(1), pp. 665–675
Li, H., Li, P., Zhang, H., Chow, Y.C.,
Wong, M.S., Pinna, S., Klamkin, J., Speck, J.S., Nakamura, S., DenBaars, S.P.,
2020. Electrically Driven, Polarized, Phosphor-Free White Semipolar (20-21)
InGaN Light-Emitting Diodes Grown on Semipolar Bulk GaN Substrate. Optics
Express, Volume 28(9), pp. 13569–13575
Ma, L., Zhao, Y., Du, M., Pei, X., Feng,
X., Sun, F., Fang, S., 2021. Phase-Error-Compensation-Based Surface Recovery
Algorithm Using Spectrum Selection for White Light Interferometry. Applied
Optics, Volume 60(21), pp. 6030–6043
Qin, L., Shi, X., Leon, A.S., 2020.
Luminance Calculation Method Accounting for Mesopic Vision and Fog Penetration
Ability. Applied Optics, Volume 59(3), pp. 683–686
Shadalou, S., Cassarly, W.J., Suleski T.J.,
2021. Tunable Illumination for LED-Based Systems Using Refractive Freeform
Arrays. Optics Express, Volume 29(22), pp. 35755–35764
Shi, L., Zhao, X., Du, P., Liu, Y., Lv, Q.,
Zhou, S., 2021. Enhanced Performance of Gan-Based Visible Flip-Chip Mini-LEDs
With Highly Reflective Full-Angle Distributed Bragg Reflectors. Optics
Express, Volume 29(25), pp. 42276–42286
Shih, H.K., Liu, C.N., Cheng, W.C., Cheng,
W.H., 2020. High Color Rendering Index of 94 in White LEDs Employing Novel
CaAlSiN3: Eu2+ and Lu3Al5O12: Ce3+
Co-Doped Phosphor-in-Glass. Optics Express, Volume 28(19), pp. 28218–28225
Singh, K.J., Fan, X., Sadhu, A.S., Lin,
C.H., Liou, F., Wu, T., Lu, Y., He, Jr., Chen, Z., Wu, T., Kuo, H., 2021.
CsPbBr3 Perovskite Quantum-Dot Paper Exhibiting a Highest 3?Db
Bandwidth and Realizing A Flexible White-Light System for Visible-Light
Communication. Photonics Research, Volume 9(12), pp. 2341–2350
Sun, C.C., You, A.H., Teo, L.L., 2022. XRD
Measurement for Particle Size Analysis of PMMA Polymer Electrolytes with SiO2.
International Journal of Technology, Volume 13(6), pp. 1336–1343
Tian, W., Dou, L., Jin, Z., Xiao, J., Li,
J., 2020. Full-Color Micro-LED Displays with Cadmium-Free Quantum Dots
Patterned by Photolithography Technology. Applied Optics, Volume 59(35),
pp. 11112–11122
Tuyet, D.T., Quan, V.T.H., Bondzior, B.,
Deren, P. J., Velpula, R.T., Nguyen, H.P.T., Tuyen, L.A., Hung, N.Q., Nguyen,
H.D., 2020. Deep Red Fluoride Dots-In-Nanoparticles for High Color Quality
Micro White Light-Emitting Diodes. Optics Express, Volume 28(18), pp.
26189–26199
Wang, W., Cheng, C., Wang, H., Lin, G.,
2020. White-Light Color Conversion With Red/Green/Violet Laser Diodes and
Yellow Light-Emitting Diode Mixing for 34.8?Gbit/s Visible Lighting
Communication. Photonics Research, Volume 8(8), pp. 1398–1408
Yu, X., Xiang, L., Zhou, S., Pei, N., Luo,
X., 2021. Effect of Refractive Index of Packaging Materials on The Light
Extraction Efficiency of COB-LEDs with Millilens Array. Applied Optics,
Volume 60(2), pp. 306–311
Yuce, H., Guner, T., Balci, S., Demir,
M.M., 2019. Phosphor-Based White LED by Various Glassy Particles: Control over
Luminous Efficiency. Optics Letter, Volume 44(3), pp. 479–482
Zhang, C., Yang, B., Chen, J., Wang, D.,
Zhang, Y., Li, S., Dai, X., Zhang, S., Lu, M., 2020. All-Inorganic Silicon
White Light-Emitting Device with An External Quantum Efficiency of 1.0%. Optics
Express, Volume 28(1), pp. 194–204
Zhang, K., Lu, H.B., Shao, L., Zheng, C.,
Zhang, Y.J., Huang, S.Y., 2021. Experimental Research on Ammonia Concentration
Detection with White Light-Emitting Diodes. Journal of Optical Technology,
Volume 88(9), pp. 548–552
