Extractive Fed-Batch Bioacetone Fermentation with Free Cell and Immobilized Clostridium Saccharoperbutylacetonicum N1-4 in a Large Extractant Volume
Corresponding email: r.f.darmayanti@gmail.com
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.5735
Darmayanti, R.F., Muharja, M., Airlangga, B., Widjaja, A., Nalawati, A.N., 2024. Extractive Fed-Batch Bioacetone Fermentation with Free Cell and Immobilized Clostridium Saccharoperbutylacetonicum N1-4 in a Large Extractant Volume. International Journal of Technology. Volume 15(4), pp. 890-902
| Rizki Fitria Darmayanti | Department of Agro-industrial Technology, Faculty of Agriculture, University of Muhammadiyah Jember, Jalan Karimata 49, 68124 Jember, Indonesia |
| Maktum Muharja | Department of Chemical Engineering, Faculty of Industrial Technology and System Engineering, Institut Teknologi Sepuluh Nopember, Surabaya 60111, Indonesia |
| Bramantyo Airlangga | Department of Chemical Engineering, Faculty of Industrial Technology and System Engineering, Institut Teknologi Sepuluh Nopember, Surabaya 60111, Indonesia |
| Arief Widjaja | Department of Chemical Engineering, Faculty of Industrial Technology and System Engineering, Institut Teknologi Sepuluh Nopember, Surabaya 60111, Indonesia |
| Ara Nugrahayu Nalawati | Department of Agro-industrial Technology, Faculty of Agriculture, University of Muhammadiyah Jember, Jalan Karimata 49, 68124 Jember, Indonesia |
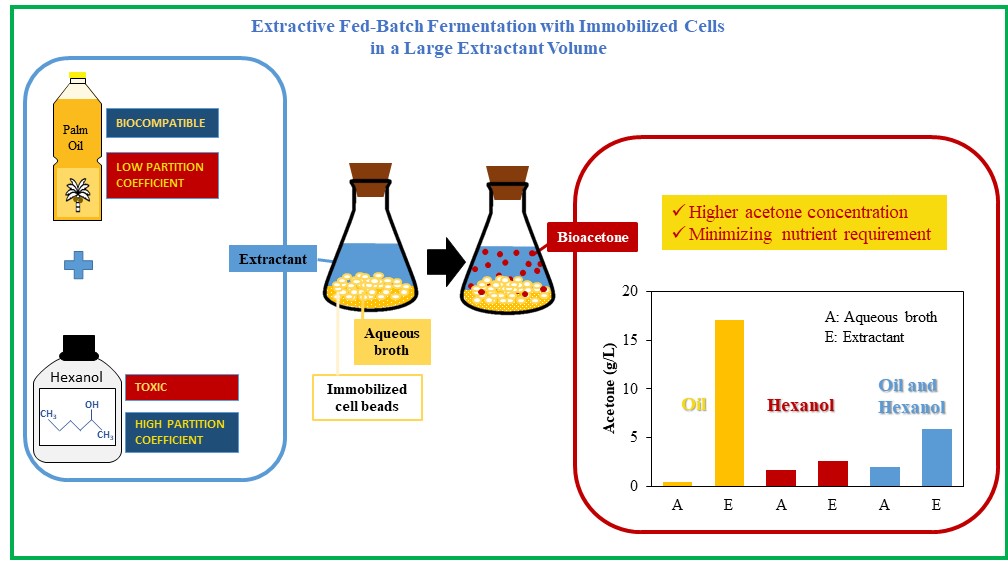
This study compared free and immobilized cells with
palm oil and hexanol as an extractant in a large volume ratio of extractant to
the broth. Fermentation was conducted using TYA (Tryptone-Yeast-Acetate) media,
glucose as the substrate and the palm oil, hexanol, and a mixture of hexanol
and palm oil with a composition of 1:1 v/v as an extractant in the fermentation
with a large volume ratio of extractant to broth of 5. The strain was
inoculated as free-suspended cells of C. saccharoperbutylacetonicum N1-4
and immobilized cells in calcium alginate. The best extractant results for the
acetone fermentation process in the ratio of extractive fermentation of free
cells and immobilized cells were using palm oil as extractants. This extractant
harvested acetone well, with the acetone concentration in the extractant at
18.41 g/L in free cell fermentation and 17.07 g/L in immobilized cell
fermentation. The maximum total acetone concentration was up to 93.72 g/L
broth. Using palm oil as an extractant in a large extractant volume combined
with immobilization is an alternative to enhance bioacetone production by
effectively reducing the toxicity effect of the products and minimizing
nutrient requirements.
Bioacetone; Cell immobilization; Clostridium saccharoperbutylacetonicum N1-4; Extractive fermentation; Free cell fermentation
Most commodity
chemicals and fuels are originated from non-renewable fossil reserves (Alimny et al., 2019). As one of the
products, acetone has a global market of about US$4 billion in 2021 (Amezquita-Ortiz et al., 2022). In
industry, acetone was utilized as a solvent and platform chemical to generate
materials such as acrylic glass and polypropylene. Moreover, in the fuel
sector, acetone was employed to boost the fuel's performance (Aguado-Deblas et al., 2020). Acetone is
generally manufactured via the cumene process as a by-product of phenol
production (Kökdemir and Acarali, 2021). The process involves propene
cracking, which is an energy-intensive and hazardous process (Liew et al., 2022). Acetone can also be
obtained from renewable resources such as lignocellulosic biomass, algae, waste
streams, industrial by-products, etc., through an
C.
saccharoperbutylacetonicum
grows at the optimum temperature of 30 oC, relatively close to
ambient temperature compared with other strains, which are mostly optimum at a
higher temperature of 37 oC, reducing the energy requirement during
fermentation. C. saccharoperbutylacetonicum N1-4 (ATCC 13564) was proven
to be able to convert many kinds of substrates to ABE, such as glucose, lactic
acid (Oshiro et al., 2011), xylose (Zheng et al., 2013), cellobiose (Noguchi et al., 2013), xylan (Al-Shorgani, Kalil, and Yusoff, 2011), hydrolyzed
agricultural waste (Wu et al., 2021; Qureshi
et al., 2008), and wood (Zheng et
al., 2013).
The major
limitation of the ABE fermentation process is the complex composition of
products with low concentrations, which resulted in the high energy demand of
downstream separation of the ABE fermentation from broth (Cai et al., 2022). In addition, the
inhibition of ABE products (i.e., butanol and acetone) limits product titer
because of the toxicity to the producer strain. This condition challenges the
economic viability of the whole ABE process (Sarangi
and Nanda, 2018). The proposed solution is the acetone recovery as fast
as it is generated, thus preventing the acetone concentration under the
inhibition threshold. Some investigated options are adsorption, gas stripping, pervaporation,
liquid-liquid extraction (LLE), etc. (Rathour et
al., 2018). The integration of ABE fermentation with LLE is an
alternative method that is under great challenge to be developed (Al-Shorgani et al., 2019; Jang et al.,
2012). Process with simpler configuration and lower energy consumption
is more preferable (Muharja et al., 2023a;
2022; 2020a). The LLE method has several astonishing advantages: it uses
a simple setup and equipment, easy to recover the products from the extractant,
and it has low energy use (Zhao et al.,
2019). Previous studies found that a large extractant volume of 5 – 10
to broth ratio significantly improved total ABE fermentation by diminishing
butanol toxicity, led to high product yield and high cell density (Darmayanti et al., 2019, 2021; Zhao et al., 2019). Enhanced acetone to
butanol product ratio in ABE fermentation up to 5:7 could be obtained by
increasing the fermentation temperature to 43.5 oC (Wu et al., 2021).
Another common
method to enhance ABE fermentation product yield by protecting the cells from
the product's harmfulness is the cell immobilization technique (Abo et al., 2019). Various cell
immobilization techniques have also been found to improve yield through the use
of high cell density in continuous fermentation mode (Menchavez
and Ha, 2019; Chang et al., 2016). Al-Shorgani
et al. (2019) reported an improvement in fermentation
productivity and stability by using an immobilized cell of Clostridium sp.
in continuous fermentation systems. Cell immobilization prevents bleeding
during the process. There are many methods for cell immobilization, namely
self-aggregation, entrapment, adsorption, or biofilm (Liu
et al., 2019). Cell immobilization via the gel entrapment method
is extensively employed due to its high mechanical properties and flexible
support structure (Gao et al., 2021).
Although research on the improvement of ABE productivity via LLE and cell
immobilization has been progressively conducted, from the literature studies,
there has been no publication focusing on the application of acetone
production.
From the aforementioned background, this study aims to develop acetone production via the ABE fermentation process with a large volume ratio of extractant to the broth. As a result, a high level of acetone production was attained by utilizing immobilized C. saccharoperbutylacetonicum N1-4 in a fed-batch system.
2.1. Materials
The chemicals used for
this experiment are hexanol (Merck, Darmstadt, Germany), palm oil, glucose,
CaCO3, (NH4)2SO4, CH3COONH4,
MgSO4.7H2O, FeSO4 -7H2O, KH2PO4,
NaCl, CaCl2, HCl and KOH (Pudak Scientific, Bandung, Indonesia),
yeast extract and tryptone (Himedia, Mumbai, India).
2.2. Microorganism Refresh
The first process is
mixing distilled water with 15 g/L of grated potato, 10 g/L of glucose, 0.5 g/L
of ammonium sulfate, and 3 g/L of CaCO3. The mixture was then heated
in boiled water for 1 hour, then cooled to room temperature. The solid was
filtered, and the suspension was sterilized and used for Potato-Glucose media.
Five spoons of C. saccharoperbutylacetonicum N1-4 ATCC 13564 sand stock
bacteria were added to 10 ml media, then heat shocked in boiling water for 1
minute. Then, it was cooled and set at room temperature for 24 hours
anaerobically.
2.3. Preculture
Media was made of
tryptone (Himedia, Mumbai, India) 6 g/L, yeast extract (Himedia, Mumbai, India)
2 g/L, CH3COONH4 3 g/L, MgSO4.7H2O
0.3 g/L, FeSO4 -7H2O 0.01 g/L, KH2PO4
0.5 g/L, and glucose 20 g/L. The pH of the solution was adjusted to 6.5 (HCl
and KOH were used as pH-adjusting agents). Refreshed media was inoculated at
10% v/v and then placed at room temperature anaerobically.
2.4. Free Cell Extractive Fermentation
The TYA media
contained tryptone 6 g/L, yeast extract 2 g/L, CH3COONH4
3 g/L, MgSO4 – 7H2O 0.3 g/L, FeSO4 -7H2O
0.01 g/L, KH2PO4 0.5 g/L, and glucose 50 g/L (Darmayanti et al., 2020). The pH of the solution was adjusted to
6.5. Palm oil, hexanol, a mixture of hexanol, and palm oil with a ratio of 1:1
was used as extractants, with an extractant-to-broth volume ratio of 5. The
working volume of fermentation was 30 mL of broth and 150 mL of extractant
(Figure 1A). It was in an incubator shaker at 160 rpm at 50°C. At the 12, 24,
48, and 72 hours, each sample was fed with 2 mL of 120 g/L glucose solution.
2.5. Extractive Fermentation with Immobilized Cells
The working volume of the fermentation was 30 mL of
broth, 30 mL of immobilized cells beads, and 150 mL of extractant (Figure 1B).
Immobilized cell beads were prepared by making sodium alginate gel (3% w/v)
containing NaCl (0.85% w/v) and preculture media of 10% v/v. The gel mixture
was then dropped in CaCl2 solution (3% w/v) to form calcium alginate
beads. The beads were filtered and used for fermentation.
Figure 1 Extractive fermentation with (a) free and (b) immobilized cells
2.6. Analysis
The reducing sugar in
the broth was measured using DNS (dinitrosalicylic acid) method (Muharja et al., 2019; 2020b). The cell density of C.
saccharoperbutylacetonicum was analyzed by measuring absorbance at 562 nm
using a UV-vis spectrophotometer; one unit of absorbance is equal to 0.246 g/L
dry cell weight. Acetone, ethanol, acetic acid, and butanol were analyzed using
gas chromatography (Darmayanti et al., 2021).
3.1. Free Cell Extractive Fermentation
This study used palm oil
and hexanol as representative natural and synthetic extractants because they
have not been reported in previous studies for bioacetone fermentation. Acetone
dissolves well in both of these organic solvents, so they were used as the
extractant for acetone in this experiment. The initial pH was adjusted at 6.5
as this is the optimum acidity for the strain to form organic acid. The pH for
free cell extractive fermentation with palm oil extractant decreased to around
5-6 (Figure 2A), while the pH in hexanol extractants and hexanol-palm oil
mixtures was at pH around 6. During the acidogenesis phase, the pH tends to
decrease due to the formation of acids, namely acetic acid, in the fermentation
with palm oil extractant. The influence of external pH is one of the key
factors involved in triggering the transition from the acidogenesis phase to
the solventogenesis phase (Capilla et al., 2024).
The addition of CaCO3
(calcium carbonate), which is a reagent that serves to buffer the degree of
acidity that can maintain the pH in the fermentation process. To achieve
optimum conditions for the growth and metabolism of acetic acid bacteria, the
environment and the state of the fermentation medium are well maintained. In
this study, the effect of the use of CaCO3 is very influential
because it maintains the pH value of each extractant. The pH in this
fermentation should not be below 5 to prevent the acid crash, which may cause
death to the strain (Capilla et al., 2022). Its low
solubility in water can cause it to neutralize acids such as acetic acid and
maintain pH at a certain level automatically (Valles
et al., 2020). For good ABE
production, the pH should be buffered between 5-6 (Han
et al., 2013), as occurred in the
extractive fermentation with palm oil as an extractant. However, using hexanol
as an extractant caused low cell growth, which consequently formed a lower acid
product and no significant pH change during fermentation.
Figure 2 Free cell extractive
fermentation pH test curve (a), cell density (b), glucose concentration (c),
and acetone concentration (d) with palm oil,
hexanol, and
hexanol-palm oil mixture as extractant. O is oil, H is hexanol, A is the aqueous phase,
and E is the extractant phase
Figure 2D shows that the
concentration of acetone in the free cell extractive fermentation was the
highest using palm oil as extractant with a value of 18.414 g/L, with a high
distribution coefficient of acetone in the palm oil–water mixture of 11.2. This
study attempted a higher temperature of 50 oC to enhance the acetone
product than butanol. As reported in the previous study with SSF of corn
stover, a higher temperature of 43.5 oC could improve the
acetone-to-butanol product ratio (Wu et al.,
2021). The substrate and nutrients in the free-cell extractive
fermentation using palm oil were directly consumed by the free cells and no
toxic barriers. Hence, the value obtained was relatively high. As the palm oil
reduced the acetone concentration effectively from the media, the acetone was
produced in a higher amount compared with the other extractant (Cai et al., 2022).
3.2. Immobilized Cell
Fermentation
Figure 3 Immobilized cell fermentation pH (a), cell density (b), glucose concentration (c), and acetone concentration (d) with
Fermentation using palm oil extractant
demonstrated a high cell concentration increase (Figure 3B); namely, the
maximum cell density is obtained at the 96th hour of 0.354 g/L.
Compared to the cell growth using free cell extractive fermentation (0.246
g/L), immobilization increased the growth. Immobilization protected the cells
inside the alginate matrix, resulting in more accumulated cells entrapped in
the beads, more than the density of free cells (Darmayanti et al., 2018). In the previous study using a low ratio
of toxic extractant to broth volume, immobilization could promote cell growth
in the alginate beads (Ye et al., 2018). In this study, a high-volume ratio of
toxic extractant to broth exposed the cells to a more extreme condition, so
they could not grow even entrapped in the calcium alginate.
Figure 3C shows that
the glucose concentration curve for immobilized cell fermentation had a stable
increase and decrease in all fermentation. The highest sugar consumption was
47.01 g/L, using palm oil as the extractant. The decrease in glucose concentration
in the hexanol extractant and the mixture of hexanol with palm oil occurred
because the bacteria were protected by the alginate and required a substrate
for growth (Muharja et al., 2023b). Although hexanol is a toxic substance,
sugar consumption was more stable than fermentation with free cells. The role
of immobilized cells in the fermentation of acetone products is to increase the
resistance of microbial cells from the influence of environmental conditions
such as pH, temperature, organic solvents, and toxic substances, thus causing
the viability of the cells to be maintained better (Ye et al., 2018).
Figure 3D shows that the
acetone concentration curve in the extractive fermentation of immobilized cells
has increased in the palm oil extractant, which is 17.069 g/L, so the palm oil
extractant is the best extractant to produce acetone products in the extractive
fermentation using immobilized cells. Cell immobilization aims to make the cell
immobile or reduce its space to move so that it inhibits its growth outside the
beads, and the substrate is used only to produce products (Hastuti et al.,
2019).
3.3. Comparison of Free
Cells and Immobilized Cells in Extractive Fermentation
Figure
4 shows the total acetone concentration to the volume of the medium based on
the method of adding glucose to food nutrients in each fermentation obtained a
high enough value for the palm oil extractant with free cell extractive
fermentation, which was 93.72 g/L. This is because the free cells are in direct
contact with the glucose substrate in the media cells. The second highest value
was found in the palm oil extractant with extractive fermentation of
immobilized cells, which was 85.81 g/L. This is because the immobilized cells
were entrapped in the alginate matrix, so the substrate mass transfer to the
immobilized cells was decelerated compared to free cells, which directly
contacted the substrate with no hindrance. However, the difference was insignificant
if immobilized cells were considered for the ease of recycling and eliminating
the nutrient requirement for the preculture of the next fermentation batch (Zhao et al., 2019). A previous study had
proven the reusability of calcium alginate for Abe fermentation with stable
results in three consecutive cycles (Kheyrandish et
al., 2015).
According
to this study's result, the extractant's biocompatibility played a stronger
role than the immobilization. In the previous study using octanol as the
extractant with a low extractant-to-broth volume ratio of 0.33, entrapment in
calcium alginate protected the cells from the toxic effect of the solvent (Ye et al., 2018). In this study, the
hexanol extractant in immobilized cells with a large volume-to-broth ratio of 5
contacted the beads in a high concentration of solvents, hampered the cell's
growth, and consequently resulted in a low acetone concentration. The use of a
biocompatible extractant (palm oil) in a large extractant-to-broth volume ratio
favored the fed-batch fermentation by eliminating the toxic effect of the
produced solvent in broth (Da-Costa-Nogueira et
al., 2021). The viability of the cells was maintained in a
productive condition, giving a high total acetone concentration reaching 93.72
g/L and 85.81 g/L.
Figure 4 Total acetone concentration per media (broth) volume. O is oil, H is
hexanol, A is the aqueous phase, and E is the extractant phase. Total acetone
concentration is the total mass of the acetone produced in the aqueous and extractant
phase divided by the total volume of broth (aqueous phase)
The data in Table 1 shows that in the
extractant and aqueous phases, there are acetone, butanol, and ethanol which
are the end products of the solventogenesis stage, and acetic acid, which is an
intermediate product of the acidogenesis stage. The fermentation in this study
using a fermentation temperature of 50 oC and acetone selective
extractant succeeded in producing acetone selectively rather than the other
products.
Table 1 Concentration of
fermentation products in each phase
|
Sample Name |
Acetone (g/L) |
Ethanol (g/L) |
Butanol (g/L) |
Acetic Acid (g/L) |
|
O-A (F) |
1.652 |
- |
- |
- |
|
O-A (I) |
0.461 |
- |
- |
- |
|
H-A (F) |
0.965 |
0.706 |
- |
0.559 |
|
H-A (I) |
1.671 |
0.212 |
0.075 |
6.492 |
|
HO-A (F) |
1.580 |
- |
- |
0.800 |
|
HO-A (I) |
2.006 |
- |
- |
- |
|
O-E (F) |
18.414 |
- |
- |
1.262 |
|
O-E (I) |
17.069 |
0.337 |
- |
- |
|
H-E (F) |
3.424 |
- |
- |
- |
|
H-E (I) |
2.566 |
- |
0.117 |
- |
|
HO-E (F) |
1.170 |
- |
0.099 |
- |
|
HO-E (I) |
5.836 |
0.155 |
- |
4.629 |
|
Information: O = Fermentation with palm
oil H = Fermentation with
hexanol HO = Fermentation with
palm oil-hexanol mixture |
A = Aqueous phase E = Extractant phase F = Fermentation with free cells I = Fermentation with immobilized cells | |||
Table 2 Comparison of acetone-enhanced ABE
fermentation
|
Fermen-tation |
Process modification |
Strain |
Tempe-rature (oC) |
Total acetone
concentration (g/L broth) |
References |
|
Batch |
Extraction using octanol with extractant to broth
ratio of 0.33 |
C. acetobutylicum |
37 |
3.33 |
(Ye et al., 2018) |
|
Batch |
Acetate addition |
C. acetobutylicum and S. cerevisiae |
37 |
7.0 |
(Luo et al., 2016) |
|
Batch |
Acetate addition |
C. acetobutylicum and S. cerevisiae |
37 |
10.5 |
(Luo et al., 2017) |
|
Fed-batch |
pH-stat with acetate and glucose co-feeding |
C.
saccharoper-butylacetonicum |
30 |
8.74 |
(Gao et al., 2016) |
|
Batch |
SSF of corn stover using thermotolerant strain |
C. acetobutylicum |
43.5 |
5.0 |
(Wu et al., 2021) |
|
Fed-batch |
Extraction using oleyl alcohol and tributyrin
mixture with extractant to broth ratio of 5 |
C.
saccharoper-butylacetonicum |
30 |
13.0 |
(Darmayanti et al., 2018) |
|
Fed-batch |
Extraction using palm oil with extractant to broth
ratio of 5 |
C.
saccharoper-butylacetonicum |
50 |
93.72 with free cells and 85.81 with immobilized
cells |
This study. |
Several studies enhancing acetone production in ABE
fermentation were reported but in a limited number (Table 2). This study using
high temperature and palm oil extractant in a large extractant-to-broth volume
ratio reached the highest total acetone concentration compared with the other
reported research. The total concentration of produced acetone was calculated
using the volume of broth as the basis because broth or media requires costly
nutrient ingredients. Besides, in industrial applications, the extractant is
recyclable, while the nutrient in the broth is consumable. A large
extractant-to-broth ratio led to a higher capacity of the broth to produce six
times more than the fermentation without extraction. High acetone concentration
was produced by the strain because of the toxicity-eliminating effect from the
use of biocompatible acetone selective extractant in a large volume. Using
extractants in a large volume is a novel option to enhance the total
concentration of product per broth so that the required nutrient during
fermentation can be minimized (Darmayanti et al., 2023). It was combined with the immobilization
of cells to ease the reuse of the cells beads to remove the preculture step
during long operations, so the nutrient required for the preculture step could
also be eliminated. These advantages are solutions to overcome the issues of
bringing ABE fermentation to a largescale production, improving the economic
feasibility of bioacetone production.
The best extractant
results for the acetone fermentation process were using free cells extractive
fermentation with palm oil extractants with a total acetone concentration of
93.72 g/L broth. The large volume of biocompatible acetone selective extractant
favored fermentation by reducing the toxicity effects of the products.
Combining the use of a large volume of extractant and immobilization is a
promising method for conducting fermentation with minimized nutrient
requirements. The research could be further investigated for the purification
of products and larger-scale applications.
The
author acknowledged the Ministry of Education, Culture, Research, and
Technology, Indonesia. We have no conflicts of interest to declare.
| Filename | Description |
|---|---|
| R1-CE-5735-20220805112242.PNG | Figure 1 |
| R1-CE-5735-20220805112439.PNG | Figure 2 |
| R1-CE-5735-20220805112458.PNG | Figure 3 |
| R1-CE-5735-20220805112704.png | Figure 4 |
Abo, B.O., Gao, M., Wang, Y., Wu, C., Wang, Q.,
Ma, H., 2019. Production of Butanol from Biomass: Recent Advances and Future Prospects.
Environmental Science and Pollution Research, Volume 26, pp. 20164–20182
Aguado-Deblas, L., Estevez, R., Hidalgo-Carrillo,
J., Bautista, F.M., Luna, C., Calero, J., Posadillo, A., Romero, A.A., Luna,
D., 2020. Acetone Prospect as an Additive to Allow the Use of Castor and
Sunflower Oils as Drop-In Biofuels in Diesel/Acetone/Vegetable Oil Triple
Blends for Application in Diesel Engines. Molecules, Volume 25(12), p.
2935.
Aisyah, A.N., Ni’maturrohmah, D., Putra, R.,
Ichsan, S., Kadja, G.T.M., Lestari, W.W., 2023. Nickel Supported on MIL-96(Al)
as an Efficient Catalyst for Biodiesel and Green Diesel Production from Crude
Palm Oil. International Journal of Technology, Volume 14(2), pp. 276–289
Alimny,
A.N., Muharja, M., Widjaja, A., 2019. Kinetics of Reducing Sugar Formation from
Coconut Husk by Subcritical Water Hydrolysis. Journal of Physics: Conference
Series, Volume 1373(012006), pp. 1-8
Al-Shorgani, N.K.N., Al-Tabib, A.I., Kadier, A.,
Zanil, M.F., Lee, K.M., Kalil, M.S., 2019. Continuous Butanol Fermentation of
Dilute Acid-Pretreated De-oiled Rice Bran by Clostridium acetobutylicum YM1. Scientific
Reports, Volume 9(1), pp. 1–13
Al-Shorgani, N.K.N., Kalil, M.S., Yusoff, W.M.W.,
2011. The Effect Of Different Carbon Sources on Biobutanol Production Using Clostridium
saccharoperbutylacetonicum N1-4. Biotechnology, Volume 10(3), pp. 1–6
Amezquita-Ortiz, J.M., Alcocer-Garcia, H.,
Contreras-Zarazua, G., Fontalvo, J., Segovia-Hernandez, J.G., 2022. Sustainable
Process Design for Acetone Purification Produced via Dehydrogenation of
2-Propanol. Industrial and Engineering Chemistry Research, Volume
61(10), pp. 3660–3671
Cai, D., Wen, J., Zhuang, Y., Huang, T., Si, Z.,
Qin, P., Chen, H. 2022. Review of Alternative Technologies for Acetone-butanol-ethanol
Separation: Principles, State-of-the-art, and Development Trends. Separation
and Purification Technology, Volume 298, pp. 121244
Capilla, Miguel, Silvestre, C., Valles, A.,
Álvarez-Hornos, F. J., San-Valero, P., Gabaldón, C., 2022. The Influence of
Sugar Composition and pH Regulation in Batch and Continuous
Acetone–Butanol–Ethanol Fermentation. Fermentation, Volume 8(5), pp. 226
Capilla, Miguel, Valles, A., San, P., Francisco,
V., Álvarez, J., Carmen, H., 2024. Solvent Production from Rice Straw by a Co -
culture of Clostridium Acetobutylicum and Saccharomyces Cerevisiae: effect of
pH control. Biomass Conversion and Biorefinery, pp. 1–13
Chang, Z., Cai, D., Wang, Y., Chen, C., Fu, C., Wang, G., Qin,
P., Wang, Z., Tan, T., 2016. Effective Multiple
Stages Continuous Acetone-butanol-ethanol Fermentation by Immobilized Bioreactors:
Making Full use of Fresh Corn Stalk. Bioresource Technology, Volume 205,
pp. 82–89
Da-Costa-Nogueira, C., de-Araújo-Padilha, C.E.,
de-Medeiros-Dantas, J.M., de-Medeiros, F.G. M., de-Araújo-Guilherme, A.,
de-Santana-Souza, D.F., dos-Santos, E.S., 2021. In-situ Detoxification
Strategies to Boost Bioalcohol Production from Lignocellulosic Biomass. Renewable
Energy, Volume 180, pp. 914–936
Darmayanti, R.F., Amini, H.W., Rizkiana, M.F.,
Setiawan, F.A., Palupi, B., Rahmawati, I., Susanti, A., Fachri, B.A., 2019.
Lignocellulosic Material from Main Indonesian Plantation Commodity as the Feedstock
for Fermentable Sugar in Biofuel Production. Asian Research Publishing
Network (ARPN) Journal of Engineering and Applied Sciences, Volume 14(20),
pp. 3524–3534
Darmayanti, R.F., Muharja, M., Tashiro, Y.,
Sonomoto, K., Zhao, T., Widjaja, A., Fahmiati, S., Halim, A., 2023. Extractive Biobutanol
Fermentation Using Immobilized Cells With Organic Acid Esters as Novel
Co-Substrates in Large Extractant Volume. Bioresource Technology Reports,
Volume 23 (101538), pp. 1-11
Darmayanti, R.F., Susanti, A., Setiawan, F.A.,
Rizkiana, M.F., Muharja, M., Aji, B.B., Prasiefa, M. G., Dewi, L.T., Yanti,
Z.A., 2021. Exploring Starch Sources for the Refreshment Process of
Acetone-Butanol-Ethanol Fermentation with Clostridium
Saccharoperbutylacetonicum N1-4. International Journal of Technology,
Volume 12(2), pp. 309–319
Darmayanti, R.F., Tashiro, Y., Noguchi, T., Gao,
M., Sakai, K., Sonomoto, K., 2018. Novel Biobutanol Fermentation at a Large Extractant
Volume Ratio Using Immobilized Clostridium Saccharoperbutylacetonicum N1-4. Journal
of Bioscience and Bioengineerinng, Volume 126(6), pp. 750–757
Darmayanti, R.F., Tashiro, Y., Sakai, K.,
Sanomoto, K., Susanti, A., Palupi, B., Rizkiana, M.F., 2020. Biobutanol
Production Using High Cell Density Fermentation in a Large Extractant Volume. International
Journal of Renewable Energy Development, Volume 9(3), pp. 431–437
Etteh, C.C., Ibiyeye, A.O., Jeland, F.B.,
Rasheed, A.A., Ette, O.J., Victor, I., 2021. Production of Biobutanol using
Clostridia Spp Through Novel ABE Continuous Fermentation of Selected Waste Streams
and industrial By-products. Scientific African, volume 12, p. e00744
Gao, H., Lu, J., Jiang, Y., Fang, Y., Tang, Y.,
Yu, Z., Zhang, W., Xin, F., Jiang, M. 2021. Material-Mediated Cell Immobilization
Technology in the Biological Fermentation Process. Biofuels, Bioproducts and
Biorefining, Volume 15(4), pp. 1160–1173
Gao, M., Tashiro, Y., Wang, Q., Sakai, K.,
Sonomoto, K., 2016. High Acetone-butanol-ethanol Production in pH-stat
co-feeding of Acetate and Glucose. Journal of Bioscience and Bioengineering.
Volume 122(2), pp. 176-182
Han, B., Ujor, V., Lai, L.B., Gopalan, V., Ezeji,
T.C., 2013. Use of Proteomic Analysis to Elucidate the Role of Calcium
Inacetone-Butanol-Ethanol Fermentation by Clostridium Beijerinckii NCIMB 8052. Applied
and Environmental Microbiology, Volume 79(1), pp. 282–293
Hastuti, N., Darmayanti, R.F., Hardiningtyas,
S.D., Kanomata, K., Sonomoto, K., Goto, M., Kitaoka, T., 2019. Nanocellulose
from Oil Palm Biomass to Enhance Microbial Fermentation of Butanol for Bioenergy
Applications. BioResources, Volume 14(3), pp. 6936–6957
Hawashi, M., Gunawan, S., Aparamarta, H.W.,
Widjaja, T., 2019. Optimization of Solid-State Fermentation Conditions for
Cyanide Content Reduction in Cassava Leaves using Response Surface Methodology.
International Journal of Technology, Volume 10(3), pp. 291–319
Hossain, M.I., Heng, T.B., Lee, C.L., Ong, T.S.,
Islam, M.T., 2022. Green Human Resource Management, Top Management Commitment,
Green Culture, and Green Performance of Malaysian Palm Oil Companies. International
Journal of Technology, Volume 13(5), pp. 1106–1114
Jang, Y.S., Malaviya, A., Cho, C., Lee, J., Lee,
S.Y., 2012. Butanol Production from Renewable Biomass by Clostridia. Bioresource
Technology, Volume 123, pp. 653–663
Kheyrandish, M., Asadollahi, M.A., Jeihanipour,
A., Doostmohammadi, M., Rismani-Yazdi, H., Karimi, K., 2015. Direct Production
of Acetone–butanol–ethanol from Waste Starch by Free and Immobilized
Clostridium Acetobutylicum. Fuel, Volume 142, pp. 129-133
Kökdemir, B., Acarali, N., 2021. A novel study on
CHEMCAD Simulation of Isopropyl Alcohol Dehydrogenation Process Development.
Journal of the Indian Chemical Society, Volume 98(3), pp. 100035
Liew, F.E., Nogle, R., Abdalla, T., Rasor, B.J.,
Canter, C., Jensen, R.O., Wang, L., Strutz, J., Chirania, P., De Tissera, S.,
Mueller, A.P., Ruan, Z., Gao, A., Tran, L., Engle, N.L., Bromley, J.C.,
Daniell, J., Conrado, R., Tschaplinski, T.J., Köpke, M., 2022. Carbon-negative Production
of Acetone and Isopropanol by Gas Fermentation at Industrial Pilot Scale. Nature
Biotechnology, Volume 40(3), pp. 335–344
Liu, J., Zhou, W., Fan, S., Xiao, Z., 2019. Study
of the Co-production of Butanol and Hydrogen by Immobilizing Clostridium Acetobutylicum
CICC8012. Energy Procedia, Volume 158, pp. 1879–1884
Luo, H., Ge, L., Zhang, J., Ding, J., Chen, R.,
Shi, Z., 2016. Enhancing Acetone Biosynthesis and Acetone-butanol-ethanol Fermentation
Performance by Co-culturing Clostridium Acetobutylicum/Saccharomyces Cerevisiae
Integrated with Exogenous Acetate Addition. Bioresource Technology,
Volume 200, pp. 111–120
Luo, H., Zhang, J., Wang, H., Chen, R., Shi, Z.,
Li, X., Ding, J. 2017. Effectively Enhancing Acetone Concentration and Acetone/butanol
Ratio in ABE Fermentation by a Glucose/acetate co-substrate System Incorporating
with Glucose Limitation and C. Acetobutylicum/S. Cerevisiae Co-culturing. Biochemical
Engineering Journal, Volume 118, pp. 132–142
Menchavez, R.N., Ha, S.H., 2019. Fed-batch Acetone-butanol-ethanol
Fermentation using Immobilized Clostridium Acetobutylicum in Calcium Alginate Beads.
Korean Journal of Chemical Engineering, Volume 36(6), pp. 909–913
Muharja, M., Darmayanti, R.F., Fachri, B.A.,
Palupi, B., Rahmawati, I., Rizkiana, M.F., Amini, H.W., Putri, D.K.Y.,
Setiawan, F.A., Asrofi, M., Widjaja, A., Halim, A., 2023b. Biobutanol
Production from Cocoa Pod Husk Through a Sequential Green Method: Depectination,
Delignification, Enzymatic Hydrolysis, and Extractive Fermentation. Bioresource Technology Reports, Volume 21, pp.
101298
Muharja, M., Fadhilah, N., Darmayanti, R.F.,
Sangian, H.F., Nurtono, T., Widjaja, A., 2020a. Effect of Severity Factor on
The Subcritical Water and Enzymatic Hydrolysis Of Coconut Husk For Reducing
Sugar Production. Bulletin of Chemical Reaction Engineering and Catalysis,
Volume 15, pp. 786–797
Muharja, M., Fadhilah, N., Nurtono, T., Widjaja,
A., 2020b. Enhancing Enzymatic Digestibility Of Coconut Husk Using
Nitrogen-Assisted Subcritical Water For Sugar Production. Bulletin of Chemical Reaction Engineering and Catalysis,
Volume 15(1), pp. 84–95
Muharja, M., Widjaja, A., Darmayanti, R. F.,
Airlangga, B., Anugraha, R.P., Fauziyah, M., Wijanarto, E., holehuddin, M.,
Khamil, A.I., 2023a. Heat Exchanger Network Analysis of The Power Plant
Industry Using Aspen Energy Analyzer Software. ASEAN Journal of Chemical
Engineering, Volume 23(1), pp. 14–27
Muharja, M., Widjaja, A., Darmayanti, R. F.,
Fadhilah, N., Airlangga, B., Halim, A., Fadilah, S.K., Arimbawa, I.M., 2022.
Subcritical Water Process for Reducing Sugar Production from Biomass:
Optimization and Kinetics. Bulletin of Chemical Reaction Engineering and
Catalysis, Volume 17(4), pp. 839–849
Muharja, M., Umam, D.K., Pertiwi, D., Zuhdan, J.,
Nurtono, T., Widjaja, A., 2019. Enhancement of Sugar Production from Coconut Husk
Based on the Impact of the Combination of Surfactant-assisted Subcritical Water
and Enzymatic Hydrolysis. Bioresource Technology, Volume 274, pp. 89–96
Niglio, S., Marzocchella, A., Rehmann, L., 2019.
Clostridial Conversion of Corn Syrup to Acetone-Butanol-Ethanol (ABE) via Batch
and Fed-batch Fermentation. Heliyon, Volume 5(3), pp. e01401
Noguchi, T., Tashiro, Y., Yoshida, T., Zheng, J.,
Sakai, K., Sonomoto, K., 2013. Efficient Butanol Production without Carbon Catabolite
Repression from Mixed Sugars with Clostridium Saccharoperbutylacetonicum N1-4. Journal
of Bioscience and Bioengineering, Volume 116(6), pp. 716–721
Oshiro, M., Hanada, K., Tashiro, Y., Sonomoto, K.,
2010. Efficient Conversion of Lactic Acid to Butanol with pH-stat Continuous Lactic
Acid and Glucose Feeding Method by Clostridium Saccharoperbutylacetonicum. Applied
Microbiology and Biotechnology, Volume 87, pp. 1177–1185
Qureshi, N., 2008. Banking on Biobutanol. Agricultural
Research, Volume 10, pp. 8–9
Rathour, R.K., Ahuja, V., Bhatia, RK., Bhatt,
A.K., 2018. Biobutanol: New era of biofuels. International Journal of Energy
Research, Volume 42(15), pp. 4532–4545
Sarangi, P.K., Nanda, S., 2018. Recent Developments
and Challenges of Acetone-butanol-ethanol fermentation. Recent Advancements in
Biofuels and Bioenergy Utilization, PP. 111-123
Su, Z., Wang, F., Xie, Y., Xie, H., Mao, G.,
Zhang, H., Song, A., Zhang, Z., 2020. Reassessment of the Role of CaCO3
in n-butanol Production from Pretreated Lignocellulosic Biomass by Clostridium Acetobutylicum.
Scientific Reports, Volume 10(1), pp. 1–10
Valles, A., Álvarez-Hornos, F.J., Martínez-Soria,
V., Marzal, P., Gabaldón, C., 2020. Comparison of Simultaneous Saccharification
and Fermentation and Separate Hydrolysis and Fermentation Processes for Butanol
Production from Rice Straw. Fuel, Volume 282, p. 118831
Veza, I., Said, M.F., Latiff, Z.A., 2021. Recent Advances
in Butanol Production by Scetone-butanol-ethanol (ABE) Fermentation. Biomass
and Bioenergy, Volume 144, pp. 105919
Wu, Y., Wang, Z., Ma, X., Xue, C., 2021. High Temperature
Simultaneous Saccharification and Fermentation of Corn Stover for Efficient Butanol
Production by a Thermotolerant Clostridium Acetobutylicum. Process
Biochemistry, Volume 100, pp. 20–25
Ye, Z., Song, J., Zhu, E., Song, X., Chen, X.,
Hong, X., 2018. Alginate Adsorbent Immobilization Technique Promotes Biobutanol
Production by Clostridium Acetobutylicum Under Extreme Condition of High Concentration
of Organic Solvent. Frontiers in Microbiology, Volume 9, pp. 1–7
Zhao, T., Tashiro, Y., Sonomoto, K., 2019. Smart Fermentation
Engineering for Butanol Production: Designed Biomass and Consolidated Bioprocessing
Systems. Applied Microbiology and Biotechnology, Volume 103, pp.
9359–9371
Zhao, T., Yasuda, K., Tashiro, Y., Darmayanti,
R.F., Sakai, K., Sonomoto, K., 2019. Semi-hydrolysate of Paper Pulp Without Pretreatment
Enables a Consolidated Fermentation System with in Situ Product Recovery for
the Production of Butanol. Bioresource Technology, Volume 278, pp. 57–65
Zheng, J., Tashiro, Y., Yoshida, T., Gao, M.,
Wang, Q., Sonomoto, K., 2013. Continuous Butanol Fermentation from Xylose with High
Cell Density by Cell Recycling System. Bioresource Technology, Volume
129, pp. 360–365
