Effect of Drying Pretreatment Methods on Amla (Emblica officinalis) Extracts Obtained Through Maceration Using Ethanol as Solvent
Corresponding email: m.dani.supardan@unsyiah.ac.id
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.5669
Raaf, A., Mulana, F., Syamsuddin, Y., Suriaini, N., Supardan, M.D., 2024. Effect of Drying Pretreatment Methods on Amla (Emblica officinalis) Extracts Obtained Through Maceration Using Ethanol as Solvent. International Journal of Technology. Volume 15(4), pp. 917-926
| Auliyaa Raaf | Doctoral Program, School of Engineering, Post Graduate Program, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Farid Mulana | Department of Chemical Engineering, Universitas Syiah Kuala, Banda Aceh 23111, Indonesia |
| Yanna Syamsuddin | 1 Department of Chemical Engineering, Universitas Syiah Kuala, Darussalam, Banda Aceh, 23111, Indonesia 2 Halal Research Center, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia |
| Nanda Suriaini | Department of Chemical Engineering, Universitas Syiah Kuala, Banda Aceh, Indonesia 23111 |
| Muhammad Dani Supardan | 1 Department of Chemical Engineering, Universitas Syiah Kuala, Darussalam, Banda Aceh, 23111, Indonesia 2 Halal Research Center, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia |
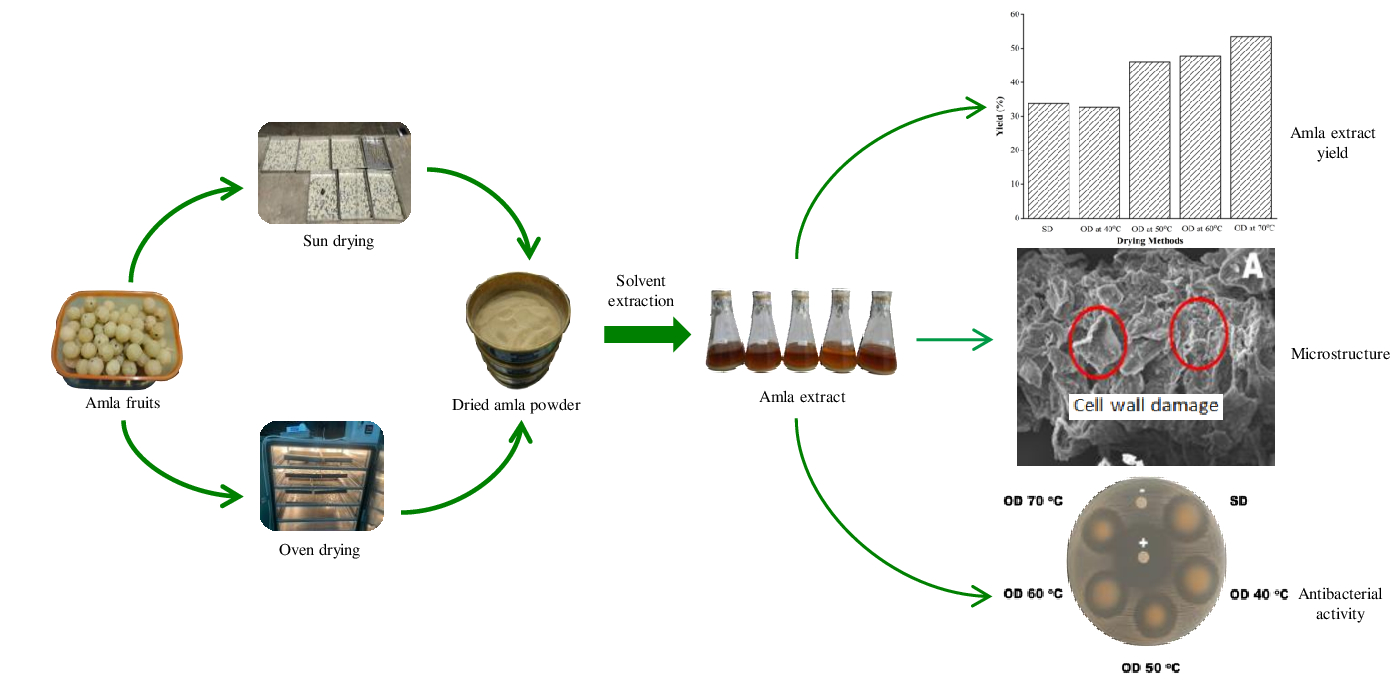
This study investigated the effects of drying methods on the yield, bioactive compounds, and antibacterial activity of amla extract. Fresh amla was oven-dried and sun-dried at different temperatures and ground into powder. Bioactive compounds were extracted from amla using maceration with ethanol as a solvent. The amla extract was used for the antibacterial susceptibility test using the agar disc diffusion method. The yield of amla extract increased with increasing drying temperature. The highest yield (53.47%) was obtained at a drying temperature of 70o C. Fourier Transform Infrared (FTIR) analysis confirmed that sun-drying and oven-drying exhibited no qualitative effect on the bioactive compound in amla extract. FTIR analysis also indicated that amla extract contains bioactive compounds, validated by phytochemical analysis. The antibacterial activity of oven-dried samples at 40 oC produced the largest inhibition zone (24.57 mm) compared to sun-drying and oven-drying at other temperatures. It can be concluded that drying temperature, especially higher temperatures, had a significant impact on the antibacterial activity of amla as its active components degraded.
Amla extract; Antibacterial activity; Bioactive compound; Drying methods
Amla (Emblica officinalis) has
long been recognized for its health benefits and has been used as a medicinal
plant and in food preparation. It is currently distributed in India, Sri Lanka,
South-East Asia, China, and Indonesia (Khan, Qais, and Ahmad,
2019)
and is available in
large quantities for food and pharmaceutical purposes. Amla has been
extensively used as a medicinal plant to treat inflammation, as an
antibacterial, and to treat cancer (Pareek and Kitinoja, 2011). A previous study has reported
that amla has high pharmaceutical activity, sparking interest due to its
potential as an antibacterial source (Jahan and Akter, 2015).
The
potential of amla as a medicinal plant is mostly due to the bioactive compounds
contained in the plant, which can treat various diseases in the human body. The
bioactive compounds include flavonoids, tannins, alkaloids, saponins, and
phenolic compounds (Arina and Harisun, 2019). Hussein, Mamman, and Mansur (2015) reported that the
Bioactive compounds,
mainly phytochemical content from amla, are extremely important for many
purposes. The phytochemical content of amla extract is heavily influenced by
how the extract is produced. Maceration is an extraction method commonly
used to extract bioactive compounds from plant materials through the use of
solvents (solid–liquid extraction) (Safdar et al.,
2017).
Ethanol is one of the most widely used solvents for the extraction of plant
bioactive compounds, such as alkaloids and
flavonoids (Pratiwi,
Utami, and Arbianti, 2020). Ethanol is also considered a food-grade solvent (Dianursanti et al.,
2020).
The
extraction of bioactive compounds from a plant begins with the drying of the
plant. Plant drying aims to produce raw
materials in simplicia, facilitating the extraction of phytochemicals with a
high yield, and facilitating plant storage for future use (Amir et al., 2021; Hasmita
et al., 2015). Additionally, drying significantly reduces volume, thereby saving on
packaging, storage, and transportation costs (Sonkar et al., 2020). Gudapaty et al.
(2010)
reported that drying affects the quality attributes of amla. The drying
pretreatment method can affect the input energy, increase the extraction yield,
and increase the quality of the extracted bioactive compounds. Thus, drying
methods must be evaluated to determine their effect on extracted bioactive
compounds.
For
continuous production of amla extract, drying techniques must be determined for
recommendation to producers. Raaf et al. (2022) reported the impact of temperature and drying methods on amla's drying
kinetics and microstructure. However, little information has been reported
regarding the effect of the drying method on bioactive compounds. Thus, this
research evaluates the effects of oven-drying (at different temperatures) and
sun-drying on the phytochemical content and antibacterial activity of amla.
2.1. Materials
The
study used amla collected near Blang Bintang, Aceh Besar. The solvent used for
the extraction process was ethanol 96% (Merck, Germany). The antibacterial
assay used nutrient agar (Oxoid, UK) and amoxicillin (Mersi, Indonesia). The
bacterial species used were Staphylococcus
aureus ATCC 25923, obtained from stock cultures of Fundament Lab Sains,
Aceh Besar.
2.2. Drying Methods
Fresh amla was washed with clean water and thinly sliced and dried. Cutting was performed to ensure that the amla samples dried evenly. The amla was sun-dried (SD) in air temperatures of 37–45 OC. For oven-drying (OD), amla was placed on a tray and dried at four different temperatures (40 OC, 50 OC, 60OC, and 70 OC). All samples were ground into a fine powder using an electric blender and screened through a mesh sieve to obtain a uniform size.
2.3. Extraction Procedure
2.4. Analysis Method
Proximate
analysis of amla powder was conducted to analyze parameters such as fat
content, carbohydrates, protein, moisture, and ash using the standard method as
per Indonesian National Standard (SNI) 01-2891-1992 procedures.
The preliminary
phytochemical evaluation of each extract was qualitatively tested to determine
the presence of flavonoids, alkaloids, tannins, saponin, quinone, steroids, and
triterpenoids. The tests were performed according to the methods reported by Guluma et
al. (2020).
A
Fourier transform infrared spectrophotometer (FTIR Shimadzu Prestige 6400) was
used to identify the functional group of amla extract. The spectra were observed
in the wavenumber region of 4000–400 cm-1. A Carl Zeiss-Bruker EVO
MA10 scanning
electron microscope (SEM) was used to observe the morphological changes of the
dried amla powder after extraction. The analysis was presented using a 1000×
magnification.
Figure 1 Illustration of the amla
extraction process using maceration
2.5. Antibacterial Assay
The
antibacterial activity of the amla extract was tested using the Kirby–Bauer
agar disk diffusion method, as reported by Zullkiflee et al. (2022). S. aureus (ATCC 25923) was used in this study. The nutrient agar
(NA) was used as the media. The NA was
compacted in a Petri dish, and the bacterial culture was inoculated and diluted
with 0.5 McFarland standard. The amla extract-soaked paper disk was drained and impregnated on the
agar plate medium surface. The plates were incubated for 24 h at 37 OC. The clear zone around the paper
disk was measured to determine the antibacterial activity. All results were
compared to distilled water as a negative control and the standard antibiotic
(amoxicillin) as a positive control.
3.1. Proximate Analysis of Dried
Amla Powder
The
proximate composition of dried amla powder is presented in Table 1. The
proximate analysis results indicate that the carbohydrate and ash values are
similar to those reported by Mishra and Mahanta (2014) for amla fruit powder; the fat and protein
content was lower. This is a result of fruit from different geographical
locations, which affects the nutritional content of the fruit, including fat
and protein, as reported by Okeke et al. (2021). The
fat content of amla ranged from 0.36% to 0.48% (Parveen and Khatkar, 2015). The protein content in amla is used by the
body for growth and maintenance. Protein also plays a role in the formation of
blood cells and antibodies that protect the body from disease and infection (Hermann, 2019). The obtained moisture content was high
(10.08%). A less than 10% moisture content must be maintained to prevent
microbial growth in dry food products (Zambrano et al.,
2019).
Thus, proper pretreatment is required to limit the amount of moisture in dried
amla powder without reducing its nutritional value.
Table 1 Proximate analysis of
dried amla powder
|
Parameter |
Content (%) |
|
Moisture |
10.08 |
|
Ash |
3.57 |
|
Fat |
0.15 |
|
Protein |
4.43 |
|
Carbohydrates |
81.77 |
3.2. Effect of Drying Methods on
Amla Extract Yield
The effect of the drying method on the yield of amla extract is presented in Figure 2. Oven-drying at 50-70oC produced a higher amla extract yield than sun-drying. The amla extract yield was slightly higher with sun-drying than with oven-drying at 40oC as the drying temperature was 40–45 oC. This indicates that drying methods have a linear effect on the increase in amla extract yield. These results are consistent with those reported by Justine et al. (2019).
The amla extract yield increased linearly with increasing drying temperature, related to the moisture content in the amla. Higher drying temperatures produced a lower moisture content in the amla (Raaf et al., 2022). Thermal drying causes cell wall damage, facilitating the release of phytochemical compounds and increasing amla extract yield (Justine et al., 2019). Cell wall damage caused by thermal drying is shown in Figure 3. Higher drying temperatures can increase cell wall damage (Figure 3B). Raaf et al. (2021) reported that the amount of water in the material at high temperatures was smaller, resulting in the stretching of the plant cell wall structure, which facilitated the rupture of the cell wall into small particles.
Figure 3 Effect of drying
temperature on the cell wall damage after extraction: A: 50 oC; B: 70 oC
Figure 4 Effect of extraction on cell wall damage: A:
before extraction (after oven-drying at 60 oC); B: after extraction
The effect of the drying method on cell wall damage after extraction is presented in Figure 5. Sun-drying (Figure 5A) and oven-drying at 40 oC (Figure 5B) have similar thermal temperatures, with a difference of 5 oC. Cell wall damage caused by thermal drying is difficult to determine. The amla extract yield was 1% greater with sun-drying than with oven-drying at 40 oC (Figure 2). Cell wall damage after extraction appeared to be greater with oven-drying at 40 oC than sun-drying. Oven-drying has a closed drying system; sun-drying has an open drying system. There is less heat loss in oven-drying than sun-drying (Babu et al. 2018). Thus, oven-drying at 40 oC (Figure 5B) observed greater cell wall damage than sun-drying (Figure 5A).
3.3. Effect of Drying Method on Phytochemical
Analysis of Amla Extract
Figure 5 Effect of drying method on cell wall damage after
extraction: A: sun-drying; B: oven-drying at 40 oC
Figure 6 FTIR spectrum of amla extract (sun-drying (SD) and oven
drying (OD) at 40–70oC)
The FTIR spectra of amla extract exhibited several main peaks. The peak
at 3652–3588 cm-1 indicates the presence of –OH (stretching)
vibration of the phenol group (Nandiyanto,
Oktiani, and Ragadhita, 2019). The C-H
vibration was found at 2964–2957 cm-1. It exhibits phenolic aromatic
compounds (Öztürk
et al., 2019). The peak at
1759–1754 cm-1 shows the ester group's C=O (stretching) vibration (Nandiyanto,
Oktiani, and Ragadhita, 2019). Peaks
at 1473–1470 cm-1, 1410–1409 cm-1, and 1257–1254 cm-1
indicate C=C-C (stretching) vibration of the aromatic ring, -OH (bending) of
the phenol group, and C-O (stretching) of the phenol aromatic ring,
respectively (Nandiyanto,
Oktiani, and Ragadhita, 2019).
Overall, the FTIR spectra exhibited good consistency with the results reported
by Firdous,
Ringø, and Elumalai (2020).
Polyphenol compounds are indicated by the functional groups of –OH, C=O, and
C-O (Raaf et
al., 2021). This ester group
indicates the presence of flavonoids (Noh, Azmin, and Amid, 2017),
saponins (Almutairi
and Ali, 2015), and tannins (Grasel,
Ferrão, and Wolf, 2016).
The results are also supported and confirmed by identification using the
reagents presented in Table 2. Flavonoids, saponins, and tannins were
qualitatively identified through FTIR and phytochemically by reagents in amla
extract under each drying condition. The results indicated that sun-drying and
oven-drying had no qualitative effect on the phytochemical compounds in amla
extract.
3.4. Antibacterial Activity
Figure 7 Inhibition of S. aureus growth by amla extract using sun-drying (SD) and oven drying (OD) at 40–70oC
The antibacterial activity of oven-dried samples (40 oC) was shown to be greater than that of other oven-dried (50 oC, 60 oC, 70 oC) and sun-dried samples. Compared with the other treatments, drying at 40 ? exhibited the greatest inhibitory activity, with an inhibition zone of 24.57 mm against gram-positive bacteria S. aureus. The results indicate that amla extract has reasonable antibacterial activity against the test microorganisms regardless of the drying method. The antibacterial activity of amla extract on S. aureus was greater than that previously reported by Kumari and Khatkar (2016).
The
inhibition zones indicated the test organisms' susceptibility to amla extract
and decreased as the amla drying temperature increased. This is reasonable; Hussein, Mamman, and Mansur (2015) reported
that M. oleifera leaf extract was
more effective at low temperatures. The presence of tannins (Khan, Qais, and Ahmad,
2019) and flavonoids (Abdullah et al., 2019) as
bioactive components is thought to be responsible for the antibacterial
activity of amla extract.
The drying method significantly affected the yield of amla extract, which was slightly greater with sun-drying than with oven-drying at 40oC and increased with increasing drying temperature. Sun-drying and oven-drying methods caused cell wall damage in dried amla powder before extraction. The extraction process also causes cell wall damage due to solvent activity, which releases phytochemical compounds from dried amla powder. FTIR and phytochemical analysis indicated that amla extract contains flavonoids, tannins, and saponins. The presence of an inhibition zone indicated that amla extract demonstrated antibacterial activity. The antibacterial activity of amla extract on S. aureus decreased with increasing amla drying temperature.
The authors are
thankful for the support of the Ministry of Education and Culture of the
Republic of Indonesia and Universitas Syiah Kuala through the PMDSU research
grant (No. 060/E5/PG.02.00.PT/2022).
Abdullah, N.A., Ja'afar, F., Yasin, H.M., Taha, H., Petalcorin, M.I.R.,
Mamit, M.H., Kusrini, E., Usman, A., 2019. Physicochemical Analyses,
Antioxidant, Antibacterial, and Toxicity of Propolis Particles Produced by
Stingless Bee Heterotrigona Itama Found in Brunei Darussalam. Heliyon, Volume
5(9), p. e02476
Ajak, S.N.F.H., Ja’afar, F., Yasin, H.M., Petalcorin, M.I.R., Usman, A.,
2020. Electrochemistry, Antioxidant Activity, and Toxicity of Unfermented Aidia borneensis Leaves Infusions. Chinese Traditional Medical Journal, Volume 3(1), pp. 1–11
Almutairi, M.S., Ali, M., 2015. Direct Detection of
Saponins in Crude Extracts of Soapnuts by FTIR. Natural Product Research, Volume 29(13), pp. 1271–1275
Amir, N., Efendy, M., Yoo, Y.J., Gozan,
M., 2021. Improved Salt Quality and Reduced Energy Consumption via Hot Air
Drying. International Journal of Technology. Volume 12(3),
pp. 592–601
Aneklaphakij,
C., Saigo, T., Watanabe, M., Naake, T., Fernie, A.R., Bunsupa, S.,
Satitpatipan, V., Tohge, T., 2021. Diversity of Chemical Structures and
Biosynthesis of Polyphenols in Nut-bearing Species. Frontiers in Plant Science, Volume 12, pp. 1–15
Arina, M.Z.I., Harisun, Y., 2019. Effect of Extraction
Temperatures on Tannin Content and Antioxidant Activity of Quercus Infectoria
(Manjakani). Biocatalysis and Agricultural Biotechnology, Volume 19, pp. 1–5
Babu, A.K., Kumaresan, G.,
Anthony-Aroul Raj, V., Velraj., R., 2018. Review of Leaf
Drying: Mechanism and Influencing Parameters,
Drying Methods, Nutrient Preservation, and Mathematical Models. Renewable and Sustainable
Energy Reviews, Volume 90, pp.
536–556
Bin Fazal, D., Ahmad, I., 2024. A Critical Analysis of
Extracts of Okra Seeds and Amla Offering Green Chemistry for Synthesis of Zinc
Oxide Nanoparticles and Their Promising Role in Reducing Crude Oil Viscosity. Materials
Today Communications, Volume 38, pp. 108172
Dianursanti, Siregar, A.R., Maeda, Y., Yoshino, T.,
Tanaka, T., 2020. The Effects of Solvents and Solid-to-Solvent Ratios on
Ultrasound-Assisted Extraction of Carotenoids from Chlorella vulgaris. International
Journal of Technology, Volume 11(5), pp. 941–950
Firdous, A., Ringø, E., Elumalai, P., 2021. Effects of
Green Tea- and Amla Extracts on Quality and Melanosis of Indian White Prawn (Fenneropenaeus indicus, Milne Edwards,
1837) During Chilled Storage. Aquaculture
and Fisheries, Volume 6, pp. 617–627
Grasel, F.S., Ferrão, M.F., Wolf, C.R., 2016. Development
of Methodology for Identification the Nature of the Polyphenolic Extract by
FTIR Associated with Multivariate Analysis. Spectrochimica
Acta Part A: Molecular and Biomolecular Spectroscopy, Volume 153, pp. 94–101
Gudapaty, P., Indavarapu, S., Korwar, G.R., Shankar, A.K.,
Adake, R.K.V., Bandi V., Kanche, S.R., 2010. Effect of Open Air Drying, LPG
Based Drier and Pretreatments on the Quality of Indian Gooseberry (aonla). Journal of Food Science and Technology, Volume 47(5), pp. 541–548
Guluma, T., Babu N.G., Teju, E., Dekebo, A., 2020.
Phytochemical Investigation and Evaluation of Antimicrobial Activities of
Brucea Antidysenterica Leaves. Chemical Data Collections, Volume 28, pp. 1–10
Hasmita, I., Adisalamun, Alam, P.N., Satriana, Mahlinda,
Supardan, M.D.,
2015. Effect of
Drying and Hydrodistillation Time on the Amount of Ginger Essential Oil. International Journal on Advanced
Science, Engineering and Information Technology, Volume 5, pp. 300–303
Hermann, J.R., 2019. Protein and the Body. Agricultural
Sciences and Natural Resources, pp. 1–4
Hussein, I.I., Mamman, M., Mansur, A., 2015. Effect of
Varying Drying Temperature on the Antibacterial Activity of Moringaoleifera
Leaf (Lam). IOSR Journal of Pharmacy and Biological Sciences, Volume 10(4), pp. 39–43
Jahan, N., Akter, S., 2015. Assessment of the Antimicrobial Activity of the
Ethanolic Extract of Phyllanthus Emblica in Combination with Different Classes
of Antibiotics against Single and Multi-Drug Resistant Strains. Journal of
Pharmacognosy and Phytochemistry, Volume 4(4), pp. 142–155
Justine,
V.T., Mustafa, M., Kankara, S.S., Go, R., 2019. Effect of Drying Methods and
Extraction Solvents on Phenolic Antioxidants and Antioxidant Activity of Scurrula ferruginea (Jack) Danser
(Loranthaceae) Leaf Extracts. Sains
Malaysiana, Volume 48 (7), pp. 1383–1393
Khan, M.S., Qais, F.A., Ahmad, I., 2019. Indian Berries
and Their Active Compounds. Elsevier Inc
Kumari, P.,
Khatkar, B.S., 2016. Assessment of Total Polyphenols, Antioxidants and
Antimicrobial Properties of Aonla Varieties. Journal of Food Science and Technology, Volume 53 (7), pp. 3093–3103
Mishra, P., Mahanta, C.L., 2014. Comparative Analysis of Functional and Nutritive
Values of Amla (Emblica Officinalis) Fruit, Seed and Seed Coat Powder. American
Journal of Food Technology, Volume 9 (3), pp. 151–161
Nandiyanto,
A.B.D., Oktiani, R., Ragadhita, R., 2019. How to Read and Interpret FTIR
Spectroscope of Organic Material. Indonesian
Journal of Science & Technology, Volume 4 (1), pp. 97–118
Noh,
C.H.C., Azmin, N.F.M., Amid, A., 2017. Principal Component Analysis Application
on Flavonoids Characterization. Advances
in Science, Technology and Engineering Systems Journal, Volume 2(3), pp.
435–440
Okeke,
H.C., Okeke, O., Nwanya, K.O., Offor, C.R., Aniobi, C.C., 2021. Comparative
Assessment of the Proximate and Mineral Composition of Cucumis sativus L. and Solanum aethiopicum L. Fruit Samples
Grown in South Eastern and North Central Regions of Nigeria Respectively. Natural Resources, Volume 12, pp. 237–249
Öztürk,
M., Bulduk, I., Korcan, S.E., Liman, R., Çoban, F.K., Karg?o?lu, M., Konuk, M.,
2018. Total Phenolics, Flavonoids Contents, Antioxidant Activity and DNA
Protective Effect of Lenten Rose (Helleborus
orientalis). Asian Journal of
Biochemistry, Genetics and Molecular Biology,
Volume 1 (4), pp. 1–12
Pareek, S., Kitinoja, L., 2011. Aonla ( Emblica Officinalis
Gaertn.). In Postharvest Biology and Technology of Tropical and Subtropical
Fruits, Woodhead Publishing, pp. 65–99
Parveen, K., Khatkar, B.S., 2015. Physico-Chemical
Properties and Nutritional Composition of Aonla (Emblica officinalis)
Varieties. International Food Research Journal, Volume 22(6), pp. 2358–2363
Pratiwi, F.A., Utami, T.S.,
Arbianti, R., 2020. Using Ultrasonic Assisted Extraction to Produce a
Bioinsecticide from Cigarette Butt Waste and Green Solvent to Control Armyworm
Infestation. International Journal of Technology, Volume 11(7),
pp. 1329–1336
Raaf, A., Putra,
T.W., Mulana, F., Syamsuddin, Y., Supardan, M.D., 2022. Investigation of
Kinetics of Amla (Emblica officinalis) Fruit Drying Process. South
African Journal of Chemical Engineering, Volume 41, pp. 10–16
Raaf,
A., Suriaini, N., Djafar, F., Syamsuddin, Y., Supardan, M.D., 2021. Effect of
Drying Temperature on the Moisture Loss, Acidity and Characteristics of Amla
Fruit. IOP
Conference Series: Earth and Environmental Science, Volume 667, pp. 1–6
Safdar, M.N., Kausar, T., Jabbar, S., Mumtaz, A., Ahad, K., Saddozai, A.A., 2017. Extraction and Quantification of Polyphenols from
Kinnow (Citrus Reticulate L.) Peel Using Ultrasound and Maceration
Techniques. Journal of Food and Drug Analysis, Volume 25(3), pp. 488–500
Sonkar, N., Rajoriya,
D., Chetana, R., Murthy K.V., 2020. Effect of Cultivars, Pretreatment and
Drying on Physicochemical Properties of Amla (Emblica officinalis)
Gratings. Journal of Food Science and Technology, Volume 57(3), pp. 980–992
Variya,
B.C., Bakrania, A.K., Patel, S.S., 2016. Emblica
officinalis (Amla): A Review for Its Phytochemistry, Ethnomedicinal Uses
and Medicinal Potentials with Respect to Moleculer Mechanisms. Pharmacological Research, Volume 111,
pp. 180–200
Zambrano, M.V., Dutta, B., Mercer, D.G., MacLean, H.L., Touchie, M.F., 2019. Assessment of Moisture Content
Measurement Methods of Dried Food Products in Small-Scale Operations in
Developing Countries: A Review. Trends in Food Science and Technology,
Volume 88, pp. 484–496
Zullkiflee, N., Taha, H., Abdullah, N.A., Hashim, F., Usman, A., 2022.
Antibacterial and Antioxidant Activities of Ethanolic and Water Extracts of
Stingless Bees Tetrigona Binghami, Heterotrigona Itama, and Geniotrigona
Thoracica Propolis Found in Brunei. Philippine Journal of Science, Volume
151(4), pp. 1455–1462
