Physical, Chemical and Electrical Conductivity Properties of Bismuth Oxide/ Rice Husk-Based Activated Carbon/Graphite Composite Prepared by the Hydrothermal Method
Corresponding email: yayuk.astuti@live.undip.ac.id
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.5624
Astuti, Y., Annisa, L., Widodo, D.S., Darmawan, A., 2024. Physical, Chemical and Electrical Conductivity Properties of Bismuth Oxide/ Rice Husk-Based Activated Carbon/Graphite Composite Prepared by the Hydrothermal Method. International Journal of Technology. Volume 15(4), pp. 987-998
| Yayuk Astuti | Department of Chemistry, Faculty of Natural Sciences and Mathematics, Universitas Diponegoro, Jl. Prof. Jacob Rais, Tembalang, Semarang, Central Java 50275, Indonesia |
| Lifiany Annisa | Department of Chemistry, Faculty of Natural Sciences and Mathematics, Universitas Diponegoro, Jl. Prof. Jacob Rais, Tembalang, Semarang, Central Java 50275, Indonesia |
| Didik Setiyo Widodo | Department of Chemistry, Faculty of Natural Sciences and Mathematics, Universitas Diponegoro, Jl. Prof. Jacob Rais, Tembalang, Semarang, Central Java 50275, Indonesia |
| Adi Darmawan | Department of Chemistry, Faculty of Natural Sciences and Mathematics, Universitas Diponegoro, Jl. Prof. Jacob Rais, Tembalang, Semarang, Central Java 50275, Indonesia |
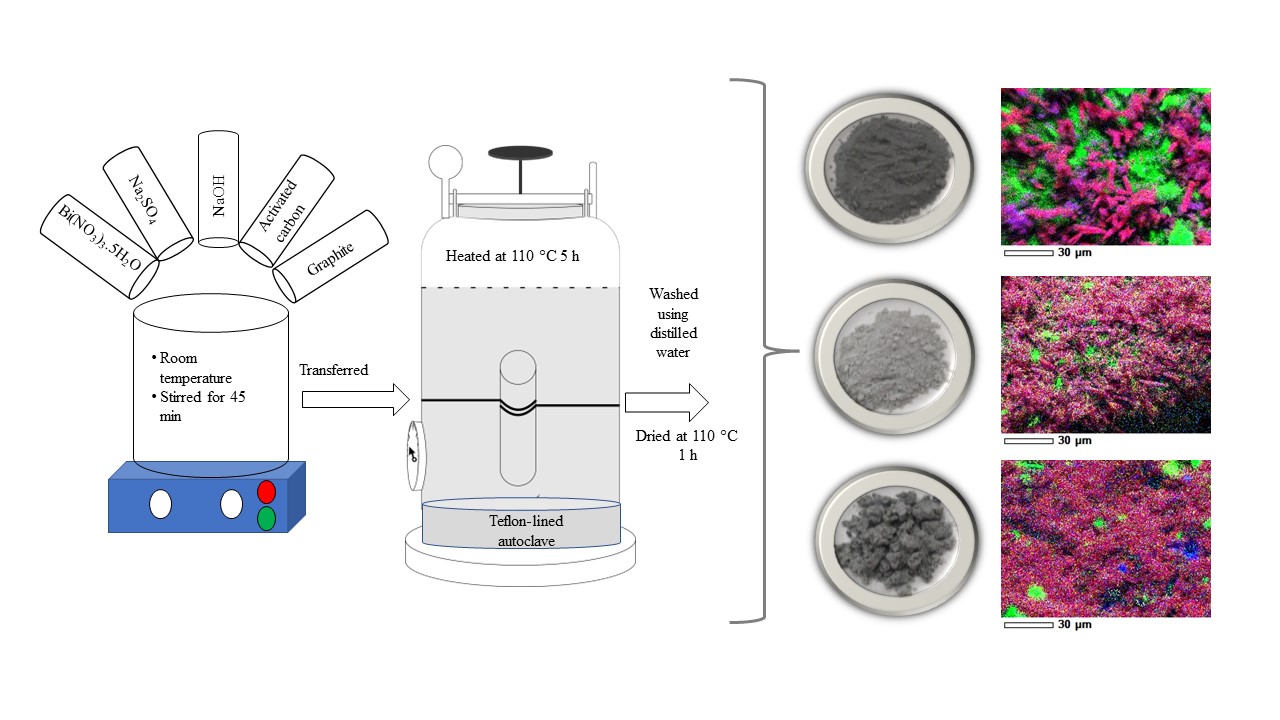
The synthesis of Bismuth
Oxide/Rice Husk-based
Activated Carbon/Graphite
(BO/RH-AC/G) composites using the hydrothermal method with varying
concentrations of bismuth nitrate pentahydrate has been conducted. The
composites are synthesized from bismuth nitrate pentahydrate, sodium sulfate,
and sodium hydroxide precursors added with activated carbon from rice husks and
graphite using the hydrothermal method at 110oC
for 5 hours. The characterization results show that the concentration of the
added bismuth nitrate pentahydrate precursor affects the physical, chemical,
and electrical properties of the resulting composites such as crystallinity,
presence of functional groups, pore and surface properties, morphology, element
distribution, thermal stability, and electrical conductivity. In the use of
smaller bismuth nitrate pentahydrate, composite has the highest crystallinity
containing mostly Bi2O3 having monoclinic crystal
structure, small pore size and high surface area. Each composite showed
different pattern of thermal decomposition. Moreover, most products have the
same rod-like morphology even though the highest precursor used resulted in
lumpy surface shape. The electrical conductivity of composites is higher than
that of pure bismuth oxide and rice husk-activated carbon.
Bismuth Oxide (BO); Composite; Graphite (G); Hydrothermal; Rice Husk-activated Carbon (RH-AC)
The battery is a source of energy supply that works by converting the
chemical energy contained in the active ingredients of the battery components
into electrical energy through electrochemical reactions of reduction and
oxidation (Reddy, 2011). Batteries have
important components that enable generate chemical reactions to create
electricity. These components include electrodes, electrolytes, and separators.
The electrode is the conductor in which the redox reaction takes place.
The battery electrode is divided into cathode and anode, which are the positive
and negative electrodes, respectively. The anode is a negative electrode, with
reference to the half-cell oxidation reaction, that releases electrons into the
external circuit (Subhan, 2011). Materials
that are able to be used as anodes must have properties that include good
charge/ion conductivity (>103 Sm-1), large energy capacity (Li
metal: 3850 mAh.g-1 (Goriparti et
al., 2014), and long life cycle, and they
also need to be easy to process, safe in use (non-toxic) and low in price.
Metal oxides (Li et al., 2021; Kim et al., 2019) have been studied intensively as somethe most promising candidates for
battery anodes due to their high theoretical capacities and low costs. One of
the metal oxides that have the potential to be used as an electrode is bismuth
oxide (Xu et al., 2022;
Nandi and Das, 2020). Expediently, bismuth oxide has high theoretical
capacity value (690 mAh.g-1), volumetric capacity value of 3765
mAh.cm-1, a potential difference of 2.8 V, is non-toxic, abundant,
and relatively cheap (Fang et al., 2017; Li et al.,
2013).
However, the problem with using bismuth oxide as a battery anode is that it
possesses low conductivity value which has a tendency to give a result of the
slow charge/ion conductivity of the battery anode. This low conductivity value,
however, is able to overcome by adding other materials that have the ability to
increase the conductivity value, namely activated carbon and graphite.
Activated carbon is an amorphous carbon consisting of a flat plate with
carbon atoms arranged and covalently bonded in a hexagonal lattice (Gilles and Loehr, 1994). Activated carbon has a
conductivity value of 6.04 S.m-1 and a large surface area between
300 m2.g-1 to 3500 m2.g-1 (Kim et al., 2016). A large surface area is
one of the advantageous factors that comprise an electrode material (Ariyanto,
Prasetyo, and Rochmadi, 2012), whereby the larger the
surface area, the greater the capacitance value (Ciszewski
et al., 2015). In addition to the surface area, the volumetric
capacity of 1770 mAh.cm-1 and a potential difference of 0.2 V (Kim et al., 2016) of activated carbon are
highly sought-after in an electrode-making material (Bijesh, Selvaraj, and Andal, 2022).
Graphite is one of the carbon core materials that act as an electrical
conductor and able to be used as an electrode material (Sari,
2015). Graphite has the characteristics of being soft, light, and
capable of conducting electricity, though graphite has a low theoretical
capacity (372 mAh.g-1) (Fang et al.,
2017).
2.1. Materials
The materials used in this study are Bi(NO3)3.5H2O
(sigma aldrich), H3PO4 (Merck), Na2SO4
(Merck), NaOH (Merck) bought from Darmstadt-Germany, rice husks, and distilled
water.
2.2. Research Procedure
2.2.1.
Rice Husk-Based Activated
Carbon Production
The production of rice
husk-based activated carbon followed the procedure reported by Arnelli, Santoso, and Astuti (2021). Clean rice husks were charred by pyrolysis at 300oC
for 10 minutes. A total of 5 grams of the carbon produced were activated by the
addition of 125 mL 60% H3PO4 and processing in the
microwave with a power of 400 W for 5 minutes. The activated carbon was then
washed with distilled water until the pH was constant. The activated carbon
that had been washed was then dried using an oven at 105oC for 5
minutes. The resulting activated carbon was destructed and sieved to a size of
100 mesh.
2.2.2.
Synthesis of Bismuth
Oxide/Rice Husk-Activated Carbon/Graphite Composite
The synthesis of BO/RH-AC/G composites
was carried out using the hydrothermal method with variations in Bi(NO3)3.5H2O
of 8, 24, and 32 mmol. For the 8 mmol BO/RH-AC/G variation, initially 8 mmol of
bismuth nitrate pentahydrate was added with 12 mmol of Na2SO4.
They were dissolved in 40 mL of distilled water and then stirred using a
magnetic stirrer for 45 minutes. Later, 40 mL of 72 mmol of NaOH was added to
the solution mixture followed by 0.5 grams of rice husk-activated carbon and
0.1 grams of graphite and the mixture was stirred. The mixture was then poured
into a stainless-steel tube and into a hydrothermal reactor, heated at 110oC
for 5 hours. At the final stage, the product was filtered and dried using an
oven at a temperature of 110oC for 1 hour, then sieved to a size of
100 mesh. The same procedure was used to synthesize the 24 mmol and 32 mmol
BO/RH-AC/G composites. The three composite products were labeled[BO/RH-AC/G]1,
[BO/RH-AC/G]2 and [BO/RH-AC/G]3 for the composites that
were synthesized with variations of 8 mmol, 24 mmol, and 32 mmol, respectively.
2.2.3.
Characterization of the Bismuth Composite Material
The characterizations of the three
composites were carried out using Fourier
Transform Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD),
Scanning Electron
Microscopy (SEM), Thermo Gravimetric Analyzer-Derivative Thermogravimetri
(TGA-DTG), Inductance,
Capacitance, and
Resistance (LCR), and Gas Sorption Analyzer (GSA) instruments. The characterization of the composite
products by FTIR (Shimadzu IRAffinity-1) was carried out in the wave number
range of 400 cm-1 - 4000 cm-1 to determine the presence
of functional groups in the resulting composite products. Analysis of composite
products using XRD (Shimadzu 7000) was done with 2theta ()= 0 to 90, to
identify the crystal structure of bismuth oxide in the synthesized composites.
Furthermore, the Scanning Electron Microscope-Energy Dispersive X-Ray (SEM-EDX)
instrument (Jeol JED 6510LA) was used to identify the surface morphology of the
sample and the compositions of the composites. A mapping test was also carried
out to determine the distribution of the elements that make up the composite
materials. The characterization of the composite products using TGA (Mettler
Toledo TGA/DSC 3+) was used to determine the thermal stability of the
composites. Meanwhile, the electrical conductivities of the composite products
were determined using an LCR meter (HIOKI 3532-50). Composite characterization
with GSA (Tristar II 3020) was carried out with N2 gas adsorbate to
determine the surface area, pore volume, and pore size of the composites.
3.1
BO/RH-AC/G Composite
The synthesis of BO/RH-AC/G composites
was initiated by reacting the precursor materials consisting of Bi(NO3)3.5H2O,
Na2SO4, and NaOH according to the procedure reported by Wu et al. (2011). Subsequently, the
solution was added with rice husk-activated carbon and graphite, homogenized,
and heated in a hydrothermal reactor.
Figure
1 Synthesized (a)
[BO/RH-AC/G]1 (b) [BO/RH-AC/G]2 and (c) [BO/RH-AC/G]3
composites
3.2 BO/RH-AC/G Composite Material
Characteristics
3.2.1. Composites Functional Groups Structures
Figure 2 FTIR spectra
of composite products, graphite, activated carbon, bismuth oxide pentahydrate
The
composite products also contain activated carbon, indicated by the presence of
the absorption of the C-O group at the wave numbers of 1118 cm-1,
1103.10 cm-1, and 1112.16 cm-1 for the [BO/RH-AC/G]1,
[BO/RH-AC/G]2, and [BO/RH-AC/G]3 products, respectively.
The absorption of the C=C group which indicates the presence of activated
carbon and graphite was also observed in the three composites at wave numbers
of 1631 cm-1, 1634.94 cm-1, and 1645.85 cm-1.
The existence of the two absorption areas is consistent with a conducted by (Sastrohamidjojo, 2018), stating that activated
carbon is characterized by a sharp absorption at wave number 1626.64 cm-1
indicating the presence of the C=C group and absorption at wave number 1039.87
cm-1 that denotes the C-O group. The absorption of the C=C group at
the wave number 1630 cm-1 also indicates the presence of graphite (Huang et al., 2017).
The identifications of the functional groups in the composite samples, pure Bi2O3,
rice husk-activated carbon, and graphite are summarized in Table 1.
3.2.2.
Crystal Structures
The XRD characterization results of the
composites are depicted in Figure 3. Composite [BO/RH-AC/G]3 has
sharp peaks at 2 theta () 21.720o; 28.896o; and 33.211o.
The peaks have similarities with the peaks in the pure Bi(NO3)3.5H2O
diffractogram, namely at 21.943o; 28.7854o, and 32.783o.
Meanwhile, the composite [BO/RH-AC/G]2 possesses no sharp and
specific peaks, meaning it is possible that the amounts of
-Bi2O3
crystals formed in this composite was small making the sample amorphous.
Figure 3
Diffractograms of the [BO/RH-AC/G]1, [BO/RH-AC/G]2, and
[BO/RH-AC/G]3 composites, rice husk-activated carbon, graphite, and
bismuth nitrate pentahydrate (BNP)
3.2.3. Morphology and Elemental
Distribution
Composite
analysis results using SEM presented in Figure 4a and b and shows that the
composite products [BO/RH-AC/G]1 and [BO/RH-AC/G]2 at
magnifications of 1000x and 5000x have almost the same rod-like morphology. The
[BO/RH-AC/G]1 composite has rod-like particles with a length of 2.72–20.9 and a width of 2.72–8.28
, while the [BO/RH-AC/G]2 is 3.63–18.18
long and 0.9–3.36
wide. The shape of the particle is distinct from the
shape of the Bi2O3 particles as reported by Yakout (2020). Meanwhile, in Figure 4c, the
composite [BO/RH-AC/G]3 has an irregular and a lumpy surface shape.
Bismuth oxide synthesized using the hydrothermal method generates rod-like
shape particles (Astuti et al., 2022b) meanwhile
other methods result in irregular shapes (Astuti et al., 2021; Astuti
et al., 2020a; 2020b). This morphology affects the value of the electrical conductivity of
bismuth oxide (Astuti et al., 2022b).
The particle shapes in one dimension including wires, rods, or tubes are the
most magnificent shape for developing the electrochemical behavior due to the
large surface areas and the shortened ion diffusion paths (Yousif et al., 2022).
Figure 5 Mapping of (a) [BO/RH-AC/G]1; (b)
[BO/RH-AC/G]2; (c) [BO/RH-AC/G]3
3.2.4. Composite Thermal Stability
The TGA-DTG curves of the [BO/RH-AC/G]1
composite (Figure 6a) shows five stages weight loss. The first and second
stages occurred at the temperatures between 200–380oC in which
around 0.2% and 13% of mass are lost and are attributed to the removal of NaOH,
NaNO3, and SO42- residues as well as the mass lost from the
transformation of the precursors into Bi2O3 (Zhang et al., 2011). In stage three, there was a further Bi2O3
formation process which occurred at temperatures between 400 – 450oC
with mass loss of around 2% (Ma et al., 2010; Christensen et al., 2003). In stage four, about 0.2% weight reduction was
observed 0.2% at 500oC, denoting the phase transition from -Bi2O3
to
-Bi2O3 (Shen et
al., 2012). The fifth stage at
640oC indicated by a weight loss of about 0.2% is identified as the
phase transition from
-Bi2O3 to
-Bi2O3
(Klinkova et al., 2007).
There are four stages of mass loss that occurred
in [BO/RH-AC/G]2 as shown in Figure 6.b. The first stage was able to
be observed at temperatures between 200–380oC where there was a
weight loss of about 9% which was able to be attributed to the removal of
residues such as NaOH, NaNO3, and SO42- and
the mass loss from the transformation from the precursors to Bi2O3
(Zhang et al., 2011). In the second stage, there was a reduction in
weight at temperatures between 400–450oC as much as 1.5%, representing the
process of further formation of Bi2O3 (Ma et al., 2010; Christensen et al., 2003). In the third stage, there was a reduction in weight of 0.5% at the
temperatures around 500oC denoting the phase transition from -Bi2O3
to
-Bi2O3 (Shen et
al., 2012). In the last stage,
at 640oC, mass loss of about 2% occurred, which is the phase
transition from
-Bi2O3 to
-Bi2O3 (Klinkova et al., 2007).
Figure 6 TGA and DTG curves
of (a) [BO/RH-AC/G]1; (b) [BO/RH-AC/G]2; (c) [BO/RH-AC/G]3
composites
The [BO/RH-AC/G]3 composites
underwent three stages of mass loss as shown in Figure 6.c. In the first stage,
there was a weight loss of 8% at temperatures between 200-275oC
which may be derived from the removal of functional groups (-COO-, -CO-, -OH-,
and hydrate) from graphite (Deng et al., 2017). The second stage was accentuated by a mass
reduction of 10% at the temperature of about 400oC which was
assigned to the transformation of the precursors to form Bi2O3
(Ma et al., 2010). In the third stage, a mass loss of 0.2% occurred at the temperature of
about 500oC which was attributed to the phase transition from (Shen et
al., 2012).
3.2.5
Composite Electrical Conductivity
The ionic and electrical conductivity of
a material is determined by its crystal structure. The electrical conductivity
values of the three composite products, pure bismuth oxide (Bi2O3),
activated carbon and graphite are presented in Table 2.
Table 2 shows that
the electrical conductivity value of pure Bi2O3 is very
low. So is the case of the rice husk-based activated carbon with its electrical
conductivity value. Meanwhile graphite as the dopant has a high conductivity
value. The three composites that have been formed have higher electrical
conductivity values than pure Bi2O3 and activated carbon
from rice husks. The rice husk-activated carbon added into the composite
functions as a matrix so that the distribution of bismuth oxide particles is
more even, which would make it easier for the electrons to flow, thus allowing
good electrical conductivity.
The [BO/RH-AC/G]3
composite product has the highest electrical conductivity value compared to the
[BO/RH-AC/G]1 and [BO/RH-AC/G]2 composites. This is
because there are more initial precursors contained, in which bismuth nitrate
pentahydrate has a high electrical conductivity value, causing the electrical
conductivity of the [BO/RH-AC/G]3 composites to be high.
The [BO/RH-AC/G]2
composite has the smallest electrical conductivity value. The value of the
electrical conductivity is influenced by the crystallinity of a material. The
more crystalline a material is, the higher its electrical conductivity it
becomes (Kim, Lee, and Jang, 2014). The XRD
data presented that the [BO/RH-AC/G]3 and [BO/RH-AC/G]1
are crystalline, while [BO/RH-AC/G]2 is amorphous. Therefore, the
electrical conductivity value [BO/RH-AC/G]2 is low.
The [BO/RH-AC/G]1 has an
electrical conductivity value in between the [BO/RH-AC/G]3 and
[BO/RH-AC/G]2. This is attributed to the successfully formed Bi2O3
confirmed through the XRD and FTIR data. The XRD data showed that the composite
[BO/RH-AC/G]1 contained Bi2O3 crystals and
the FTIR spectra confirmed the presence of the Bi-O group with the highest
intensity.
3.3.6 Pore and Surface Properties
Figure 7 Nitrogen adsorption-desorption isotherm
graphs of (a) [BO/RH-AC/G]1; (b) [BO/RH-AC/G]2; and (c)
[BO/RH-AC/G]3
Table 3
shows that the more the bismuth nitrate pentahydrate precursor is used, the
larger the pore size, the smaller the pore volume, and thus the smaller the
surface area. This is because a higher amount of Bi (NO3)3.5H2O in the composite
would mean that more activated carbon would be covered resulting in less number
of pores, as activated carbon has a large surface area of 18.83 m²/g. Based on
the resulting data, [BO/RH-AC/G]1 has the largest surface area and
[BO/RH-AC/G]3 has the smallest. The data is supported by the XRD
data (Figure 3), in which [BO/RH-AC/G]3 composite formed still
contained a lot of bismuth nitrate pentahydrate.
Larger pore size allows for faster ion transport (Hu et al., 2016). If the pore size is
large, the number of pores become less and thus generate lower resistance. This
allows more ions to flow more, resulting in a higher electrical conductivity
value. This is in accordance with the LCR data (Table 2) in which [BO/RH-AC/G]3
has the highest electrical conductivity.
The
physical, chemical, and electrical properties of BO/RH-AC/G composite prepared
by the hydrothermal method are influenced by the amount of bismuth nitrate
pentahydrate precursor added. A real difference is seen in the formation of
bismuth oxide (Bi2O3) in the resulting composite. In the
use of 8 mmol bismuth nitrate pentahydrate, the composite has the highest
crystallinity. The high amount of the precursor added also causes the composite
to tend to clump. Regarding the thermal stability as well as pore and surface
properties, each composite showed successively different patterns of thermal
decomposition and surface area and pore size. Meanwhile, the nature of the
electrical conductivity indicates that the high amount of precursor added
causes the electrical conductivity value to be higher, which may be promoted
from the precursor itself.
The authors fully acknowledged the
Institute for Research and Community Services (LPPM) of Diponegoro University
for the research funding through the World Class Research Universitas
Diponegoro (WCRU) 2021 scheme with the grant No.118-12/UN7.6.1/PP/2021.
Ariyanto, T., Prasetyo, I., Rochmadi,
R., 2012. The
Effect of Pore Structure on the Capacitance of Supercapacitor Electrodes Made
of Carbon Nanopores. Reaktor, Volume
14(1), pp. 25–32
Arnelli, Santoso, B., Astuti,
Y., 2021.
Modification of Activated Carbon of Rice Husk
using HDTMA-Br (SMAC) Surfactant
as Nitrite Ion (NO2?) Adsorbent. In: Journal
of Physics: Conference Series, IOP Publishing, Volume 1943(1), p. 012159
Astuti, Y., Amri, D., Widodo, D.S., Widiyandari, H., Balgis, R., Ogi, T., 2020a. Effect of Fuels on the Physicochemical
Properties and Photocatalytic Activity of Bismuth Oxide, Synthesized using
Solution Combustion Method. International Journal of Technology Volume 11(1), pp. 26–36
Astuti, Y., Aprialdi, F., Haryanto, I., 2019. Synthesis of Activated Carbon/Bismuth
Oxide Composite and its Characterization for Battery Electrode.
In: IOP Conference Series: Materials Science and
Engineering, IOP Publishing, Volume
509(1), p. 012153
Astuti, Y., Elesta, P.P., Widodo, D.S., Widiyandari, H., Balgis, R., 2020b. Hydrazine and Urea Fueled-solution
Combustion Method for Bi2O3 Synthesis: Characterization of Physicochemical Properties and Photocatalytic Activity. Bulletin of Chemical Reaction
Engineering and
Catalysis, Volume 15(1),
pp. 104–111
Astuti, Y., Fauziyah, A., Nurhayati, S., Wulansari, A.D., Andianingrum, R., Hakim, A.R., Bhaduri, G., 2016.
Synthesis of ?-Bismuth Oxide
using Solution Combustion Method and its Photocatalytic Properties. In: IOP
Conference Series: Materials Science and Engineering, IOP Publishing, Volume 107(1), p. 012006
Astuti, Y., Listyani, B.M., Suyati, L.,
Darmawan, A.,
2021. Bismuth Oxide Prepared by Sol-Gel Method: Variation of Physicochemical
Characteristics and Photocatalytic Activity Due to Difference in Calcination
Temperature. Indonesian Journal of Chemistry, Volume 21(1), pp. 108–117
Astuti, Y., Mei, R., Darmawan,
A., Arnelli, A., Widiyandari, H., 2022a. Enhancement of Electrical
Conductivity of Bismuth Oxide/Activated Carbon Composite. Scientia Iranica, Volume 29(6), pp. 3119–3131
Astuti, Y., Musthafa, F., Arnelli, A.,
Nurhasanah, I.,
2022b. French Fries-Like Bismuth Oxide: Physicochemical Properties, Electrical
Conductivity and Photocatalytic Activity. Bulletin of Chemical Reaction
Engineering and Catalysis,
Volume 17(1),
pp. 146–156
Bandyopadhyay, S., Dutta, A.,
2017. Thermal, Optical and Dielectric Properties of Phase Stabilized
?–Dy-Bi2O3 Ionic Conductors. Journal of Physics and
Chemistry of Solids, Volume 102,
pp. 12–20
Bartonickova, E., Cihlar, J.,
Castkova, K., 2007.
Microwave-assisted Synthesis
of Bismuth Oxide. Processing and Application of
Ceramics, Volume 1(1-2),
pp. 29–33
Bijesh, P., Selvaraj, V., Andal, V., 2022. A Review on Synthesis and Applications of Nano Metal
Oxide/porous Carbon Composite. Materials Today: Proceedings,
Volume 55, pp.
212–219
Byrappa, K., Yoshimura,
M., 2000. Hydrothermal Growth of Some Selected
Crystals. In Handbook of
Hydrothermal Technology, Nowrwich: William Andrew Publishing, pp. 198–314
Christensen, A. N., Jensen,
T.R., Scarlett, N.V., Madsen, I.C., Hanson, J.C., Altomare, A., 2003. In-situ X-ray Powder Diffraction
Studies of Hydrothermal and Thermal Decomposition
Reactions of Basic Bismuth
(iii) Nitrates in the Temperature Range 20–650 C. Dalton Transactions, Volume 16,
pp. 3278–3282
Ciszewski, M., Mianowski, A., Szatkowski, P., Nawrat, G.,
Adamek, J., 2015.
Reduced Graphene Oxide–bismuth Oxide Composite
as Electrode Material for Supercapacitors. Ionics, Volume 21(2), pp. 557–563
Deng, Z., Liu, T., Chen, T., Jiang, J., Yang, W., Guo, J., Zhao, J., Wang, H.,
Gao, L.,
2017. Enhanced Electrochemical Performances of Bi2O3/rGO
Nanocomposite via Chemical Bonding as Anode Materials
for Lithium Ion Batteries.
American Chemical Society (ACS) Applied Materials and Interfaces,
Volume 9(14),
pp. 12469–12477
Fang, W., Fan, L., Zhang, L., Zhang, Q., Yin, Y., Zhang, N., Sun, K., 2017. Synthesis of Carbon Coated
Bi2O3 Nanocomposite
Anode for Sodium-ion Batteries. Ceramics International, Volume 43(12), pp. 8819–8823
Gilles, D.G., Loehr,
R.C., 1994. Waste Generation
and Minimization in Semiconductor Industry. Journal of Environmental
Engineering, Volume 120(1),
pp. 72–86
Goriparti, S., Miele, E., De
Angelis, F., Di
Fabrizio, E., Zaccaria, R.P., Capiglia, C., 2014. Review on Recent Progress
of Nanostructured Anode Materials
for Li-ion Batteries. Journal of
Power Sources, Volume 257,
pp. 421–443
Gupta, S., Aberg, B.,
Carrizosa, S.B.,
2016. Hydrothermal Synthesis of Vanadium Pentoxides–Reduced Graphene Oxide
Composite Electrodes for Enhanced Electrochemical Energy Storage. Materials Research Society (MRS) Advances,
Volume 1(45),
pp. 3049–3055
Hu, J., Sun,
C.-F., Gillette, E., Gui,
Z., Wang, Y., Lee, S.B., 2016. Dual-template Ordered Mesoporous
Carbon/Fe2O3
Nanowires as Lithium-ion Battery Anodes.
Nanoscale, Volume 8(26),
pp. 12958–12969
Huang,
Z., Li, Z., Zheng, L., Zhou, L., Chai, Z., Wang, X., Shi, W., 2017. Interaction
Mechanism of Uranium (VI) with Three-dimensional Graphene Oxide-chitosan Composite:
Insights from Batch Experiments, Infrared (IR), X-ray Photoelectron Spectroscopy
(XPS), and Extended X-ray Absorption Fine Structure Spectroscopy (EXAFS) Spectroscopy.
Chemical Engineering Journal, Volume 328, pp. 1066–1074
Kim,
M., Lee, C., Jang, J., 2014. Fabrication of Highly Flexible, Scalable, and High-performance
Supercapacitors using Polyaniline/Reduced Graphene Oxide Film with Enhanced Electrical
Conductivity and Crystallinity. Advanced Functional Materials, Volume 24(17),
pp. 2489–2499
Kim,
T., Jo, C., Lim, W.-G., Lee, J., Lee, J., Lee, K,-H., 2016. Facile Conversion
of Activated Carbon to Battery Anode Material using Microwave Graphitization. Carbon,
Volume 104, pp. 106–111
Kim,
T., Song, W., Son, D.Y., Ono, L.K., Qi, Y., 2019. Lithium-ion Batteries: Outlook
on Present, Future, and Hybridized Technologies. Journal of Materials
Chemistry A, Volume 7(7), pp. 2942–2964
Klinkova,
L.A., Nikolaichik, V.I., Barkovskii, N.V., Fedotov, V.K., 2007. Thermal Stability
of Bi2O3. Russian Journal of Inorganic Chemistry, Volume
52(12), pp. 1822–1829
Li,
Q., Li, H., Xia, Q., Hu, Z., Zhu, Y., Yan, S., Ge, C., Zhang, Q., Wang, X., Shang,
X., Fan, S., Long, Y., Gu, L., Miao, G.-X., Yu, G., Moodera, J.S., 2021. Extra Storage
Capacity in Transition Metal Oxide Lithium-ion Batteries Revealed by in Situ Magnetometry.
Nature Materials, Volume 20(1), pp. 76–83
Li,
Y., Trujillo, M.A., Fu, E., Patterson, B., Fei, L., Xu, Y., Deng, S., Smirnov,
S., Luo, H., 2013. Bismuth Oxide: A New Lithium-ion Battery Anode. Journal
of Materials Chemistry A, Volume 1(39),
pp. 12123–12127
Ma,
M.-G., Zhu, J.-F., Sun, R.-C., Zhu, Y.-J., 2010. Microwave-assisted Synthesis
of Hierarchical Bi2O3 Spheres Assembled from Nanosheets with Pore Structure. Materials
Letters, Volume 64(13), pp. 1524–1527
Nandi,
S., Das, S.K., 2020. An Electrochemical Study on Bismuth Oxide (Bi2O3)
as an Electrode Material for Rechargeable Aqueous Aluminum-ion Battery. Solid
State Ionics, Volume 347, p. 115228
Reddy,
T.B., 2011. Linden's Handbook of Batteries. Mcgraw-hill Education, New
York
Sari,
K., 2015. Manufacturing Lithium Batteries Using Active Mesocarbon Microbead
(MCMB) As Anode With Variation in Weight Percentage of Solvent N, N-Dimethyl
Acetamide (DMAC). University of North Sumatra Institutional Repository
Sastrohamidjojo,
H., 2018. Dasar-Dasar Spektroskopi (Spectroscopy Basics). Gadjah Mada
University Press
Shen,
Y.D., Li, Y.W., Li, W.M., Zhang, J.Z., Hu, Z.G., Chu, J.H., 2012. Growth of Bi2O3
Ultrathin Films by Atomic Layer Deposition. The Journal of Physical
Chemistry C, Volume 116(5), pp. 3449–3456
Subhan,
A., 2011. Fabrication and Characterization of Li4Ti5O12
for Lithium Ceramic Battery Anode Material= Synthesis and Characterization of
Li4Ti5O12 as Anode Material for Lithium Ceramic
Battery. University of Indonesia Thesis, University of Indonesia Library
Wu,
C., Shen, L., Huang, Q., Zhang, Y.-C., 2011. Hydrothermal Synthesis and Characterization
of Bi2O3 Nanowires. Materials Letters, Volume 65(7),
pp. 1134–1136
Xu,
M., Niu, Y., Teng, X., Gong, S., Ji, L., Chen, Z., 2022. High-capacity Bi2O3
Anode for 2.4 V Neutral Aqueous Sodium-ion Battery-supercapacitor Hybrid Device
Through Phase Conversion Mechanism. Journal of Energy Chemistry, Volume 65,
pp. 605–615
Yakout,
S. M., 2020. ?-Bi2O3/?-Ni(OH)2 Composites: Effective
Solar Light Photocatalysts for Organic Pollutants Degradation. Ceramics
International, Volume 46(14), pp. 22504–22512
Yousif,
Q. A., Ranjeh, M., Ghiyasiyan-Arani, M., Al-Nayili, A., Monsef, R., Salavati-Niasari,
M., 2022. Morphology Engineering of LiFeO2 Nanostructures Through Synthesis
Controlling for Electrochemical Hydrogen Storage Inquiries. Fuel, Volume
313, p. 123025
Zhang,
L., Hashimoto, Y., Taishi, T., Nakamura, I., Ni, Q.-Q., 2011. Fabrication of Flower-shaped
Bi2O3 Superstructure by a Facile Template-free Process. Applied
surface science, Volume 257(15), pp. 6577–6582
