Application of a Polypyrrole Sensor Array Integrated into a Smart Electronic Tongue for the Discrimination of Milk Adulterated with Sucrose
Published at : 18 Jan 2023
Volume : IJtech
Vol 14, No 1 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i1.5613
Arrieta, A., Barrera, I., Mendoza, J., 2023. Application of a Polypyrrole Sensor Array Integrated into a Smart Electronic Tongue for the Discrimination of Milk Adulterated with Sucrose. International Journal of Technology. Volume 14(1), pp. 90-99
| Alvaro Arrieta | Department of Biology and Chemistry, University of Sucre (Universidad de Sucre), Road 28 No. 5-267 Red Door neighborhood, Sincelejo, postal code 700001, Colombia |
| Isora Barrera | Mercedes Abrego Educational Institution, Diag. 6-119, Tv. 1 #6-1, Monteria, postal code 230004, Colombia |
| Jorge Mendoza | Department of Mechanical Engineering, University of Córdoba, Montería, postal code 230002, Colombia |
This work presents the use of a smart electronic tongue for
discriminating adulterated milk samples with various concentrations of sucrose.
The smart electronic tongue was integrated by a voltammetric sensor array from
polypyrrole doped with various doping agents, a portable multi-potentiostat
controlled by a smartphone with an Android application. Sucrose concentrations
ranging from 1% to 20% were used to adulterate the tested samples. The sensor
array was optimized to perform measurements on milk while maintaining good
performance in terms of stability and signal quality. The sensor array was
prepared by chronoamperometric electropolymerization of pyrrole with different
doping agents, varying polymerization time (from 50 to 300 s), and
concentration of monomer (from 0.10 to 0.40 M) and doping agent (from 0.05 to
0.30 M). The optimization process results demonstrated that the parameters
polymerization time, monomer concentration, and doping agent concentration
affect the stability of the signals in the sensors, allowing for the
establishment of adequate conditions to guarantee maximum stability through an
experimental design. Thus, values of 0.10 M for monomer concentration, 0.05 to
0.10 M for doping agent concentration, and 50 to 70 s for polymerization time
were established. The measurements taken with the smart electronic tongue on
the milk samples allowed a principal component analysis to classify the samples
in the plane of the first two principal components. Principal components 1 and
2 registered a variance of 93.39% (78.68% and 14.71%, respectively), indicating
a high degree of information registered by the sensor array. It could be
concluded that the array of optimized polypyrrole sensors allows sufficient
information to be recorded through measurements made with the smart electronic
tongue to discriminate adulterated milk samples with different sucrose
concentrations.
Adulteration; Milk; Polypyrrole; Smart electronic tongue; Sucrose
Milk is one of the most important natural foods for humans due to its high nutritional value. The United Nations Food and Agriculture Organization (FAO) considers it a complete and irreplaceable food for humans due to its nutritional composition (Guetouache et al., 2014). Due to this, its consumption worldwide has increased significantly as it is considered a staple first need product in the daily diet, either as milk or any of its derivatives (Wang et al., 2020). Being a product of high consumption, it is necessary to monitor its quality and safety in order to avoid serious health problems.
An alternative to control the quality of milk
relies on four very substantial guidelines: constituents of milk, bacterial
content, its appearance, and contains adulterants. The adulteration of milk is
undoubtedly one of the most serious issues in the industry. One of the most
common practices on producer farms is the addition of water and chemicals such
as sucrose to increase profits (Yang et al., 2020). The development of new markets as a consequence of free
trade agreements between different milk-producing countries obligates
governments and the dairy industry to provide excellent quality products; as a
result, it is critical to identify foreign or rare substances in the standard
composition of milk. The main tools used for the detection of adulterants are
traditional chemical methods such as volumetric methods, infrared spectroscopy,
atomic absorption spectroscopy, liquid chromatography-mass spectrometry, and
nuclear magnetic resonance, among others (Dos-Santos et al.,
2012; Azad & Ahmed,
2016; Song et al., 2020). All these
techniques have been demonstrated to be effective in milk analysis. However,
these techniques are typically performed with expensive equipment, highly
qualified personnel, require sample pre-treatment, and lengthy analysis times.
They cannot be performed in situ due to the equipment's large volume and
weight.
The
electronic tongue can be defined as an analytical instrument, which has proved
its analytical capacity in various fields of the food industry and has been
used to analyze wines, beers, coffee, tea, honey, and milk, which have grown in
popularity in recent years. This type of device operates on systems comprised
of three major components: a sensor array with cross selectivity, an electronic
multichannel measurement device, and a data analysis unit utilizing
multivariate or pattern recognition techniques (Vlasov et al.,
2005). The electronic tongue devices
developed were based on different analytical principles, and various sensor
arrays have been used (Facure et al., 2020; Zabadaj
et al., 2018; Lipkowitz et al., 2018; Arrieta et al., 2020). However, electronic tongues based on electrochemical
measurements have received widespread acceptance from the scientific community
and the industry, particularly those based on potentiometric and voltammetric
sensor arrays (Lipkowitz et al., 2018; Arrieta et al., 2020;
Oroian & Ropciuc, 2019).
The
main systems of potentiometric electronic tongues reported the use of selective
ion or non-selective electrodes elaborated with various types of materials and
have been used in many applications and have shown to be effective in the
analysis of food products (Oroian & Ropciuc, 2019; Borges et al., 2018; Witkowska
& Kubota, 2016). However, these electronic
tongues are limited to the detection of charged chemical species (of ionic
nature); they are also highly sensitive to electronic noise and require a high
cost in terms of electronic instrumentation and data acquisition.
On
the other hand, voltammetric electronic tongues work with electrodes o sensors
made of noble metals (platinum, gold, iridium, rhodium, and palladium) or
modified electrodes (Arrieta et al., 2020; Alcañiz et al., 2012; Wei et al., 2013). In contrast to potentiometric sensors, they are not
limited to detecting charged species and possess desirable properties such as
versatility, robustness, and simplicity (Hayat et al.,
2019; Khalil et al., 2018).
Nevertheless, systems based on metallic electrodes offer poorly resolved
voltammetric signals with little information because the curves do not
represent defined electrochemical processes by the concept (Winquist
et al., 2005). For this reason, several
research groups have focused on improving and perfecting those voltammetric
systems by applying various sensor modification strategies whose signals
include better-defined electrochemical processes in order to obtain more
information from the analyzed medium. Sensors have been modified using
phthalocyanines, perylene derivatives, polypyrrole, and other substances (Arrieta
et al., 2020; Medina-Plaza et al., 2015).
The
miniaturization and modification of voltammetric sensors are common practices
in analytical chemistry (Katseli et al., 2020). However,
very few works that focus on the modification and miniaturization of sensor
arrays for electronic tongues in such a way that they allow portability and
extend their field of application to in situ analysis. This work has presented
the application of an array of miniaturized and modified polypyrrole
voltammetric sensors in analyzing milk adulterated by sucrose. The study of
milk in situ is crucial in the dairy industry because it prevents harmful or
adulterated milk from being carried to the plant. Currently, there is no
technology of this type. Hence, this research investigates the possibility of
implementing the technology to detect milk adulteration using a smart
electronic tongue in our laboratory, which is equipped with a miniaturized
sensor array coupled to a portable electronic device based on PSoC microchip
technology and controlled by a Smartphone.
2.1. Reagents and Materials
The following reagents were used: pyrrole, sodium dodecylbenzene
sulfonate, sodium sulfate, ammonium persulphate, potassium ferrocyanide,
p-toluenesulfonic acid, anthraquinone-2,6-disulfonic acid disodium salt,
lithium perchlorate, and sucrose. All the reagents used were of analytical
quality and were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). The
solutions were prepared using ultrapure water of milli-Q quality (18 M? cm-1).
The samples analyzed were produced from the same sample of raw milk from Zebu
cows (Bos taurus indicus). For this, an unadulterated sample (0%) and
sucrose-adulterated samples (1%, 2%, 3%, 4%, 5%, 10%, and 20%) were taken for
this purpose. There were five replicates for each sample, for a total of 40
samples (8 concentrations x 5 replicates each).
2.2. Smart Electronic Tongue Setup
The smart electronic tongue developed in our
laboratory consisted of a polypyrrole voltammetric sensors array and a portable
multi-potentiostat controlled by a smartphone. The sensor array was comprised
of seven electrodes or voltammetric sensors of polypyrrole (PPy) doped with
seven different doping agents (i.e., counterions): PPy/DBS (PPy doped with
sodium dodecylbenzene sulfonate), PPy/SO4 (PPy doped with sodium sulfate),
PPy/SF (PPy doped with ammonium persulphate), PPy/FCN (PPy doped with potassium
ferrocyanide), PPy/TSA (PPy doped with p-toluenesulfonic acid), PPy/AQDS (PPy
doped with anthraquinone-2,6-disulfonic acid disodium salt), and PPy/PC (PPy
doped with lithium perchlorate). The preparation of the sensor array was
conducted by chronoamperometric electropolymerization of pyrrole
at 0.8 V, using an EG&G 2273 PAR potentiostat/galvanostat, controlled by
the PowerSuite software. The PPy with each dopant was electrodeposited on
graphite substrates arranged in a circular shape using an AC9C card of BVT
Technologies.
The polymerization process was optimized by applying
an experimental design in which the variables of greatest significance were
evaluated to achieve the optimal parameters of polypyrrole synthesis in the
elaboration of each sensor in the array. The experimental process corresponds
to a 33-factorial design (three factors and three levels), totaling 27
treatments. The three factors were pyrrole concentration (0.10 M, 0.20 M, and
0.40 M), polymerization time (50 s, 100 s, and 300 s), and doping agent
concentration (0.05 M, 0.10 M, and 0.30 M). The portable multi potentiostat was
made on a FREESOC card using a PSoC 5LP microchip which was programmed with the
PSoC creator software. This electronic device was designed to record the
voltammetric signals of the seven sensors on the array simultaneously through
seven measurement channels. In addition, a Bluetooth card was also included for
data transmission to a Smartphone equipped with an Android application designed
for device control and data recording. Details on electrochemical
polymerization techniques and electronic device development have previously
been reported (Arrieta et al., 2015; Arrieta et al., 2018; Arrieta
& Fuentes, 2016).
2.3. Measurement
Milk samples were measured at room temperature on 10
mL samples. The voltammetric signals were recorded at a sweep speed of 100 mV s-1
in a potential range of -1.0 V to 0.5 V with an initial potential of 0.0 V.
Each sensor generated a voltammogram (i.e., voltammetric signal) of each
sample, which was made up of 100 current data, allowing for 700 data points
with the entire sensor array, each of which was a variable in the data matrix
in each sample. When all samples were analyzed, a matrix of 28 000 data (700
variables x 40 samples) was constructed. The obtained matrix was analyzed using
the principal components analysis method to determine the discrimination
ability of the smart electronic tongue on this type of sample.
Once the sensor array was
elaborated, it was integrated into the device to test its proper functioning
and the correct registration of the voltammetric signals through the seven
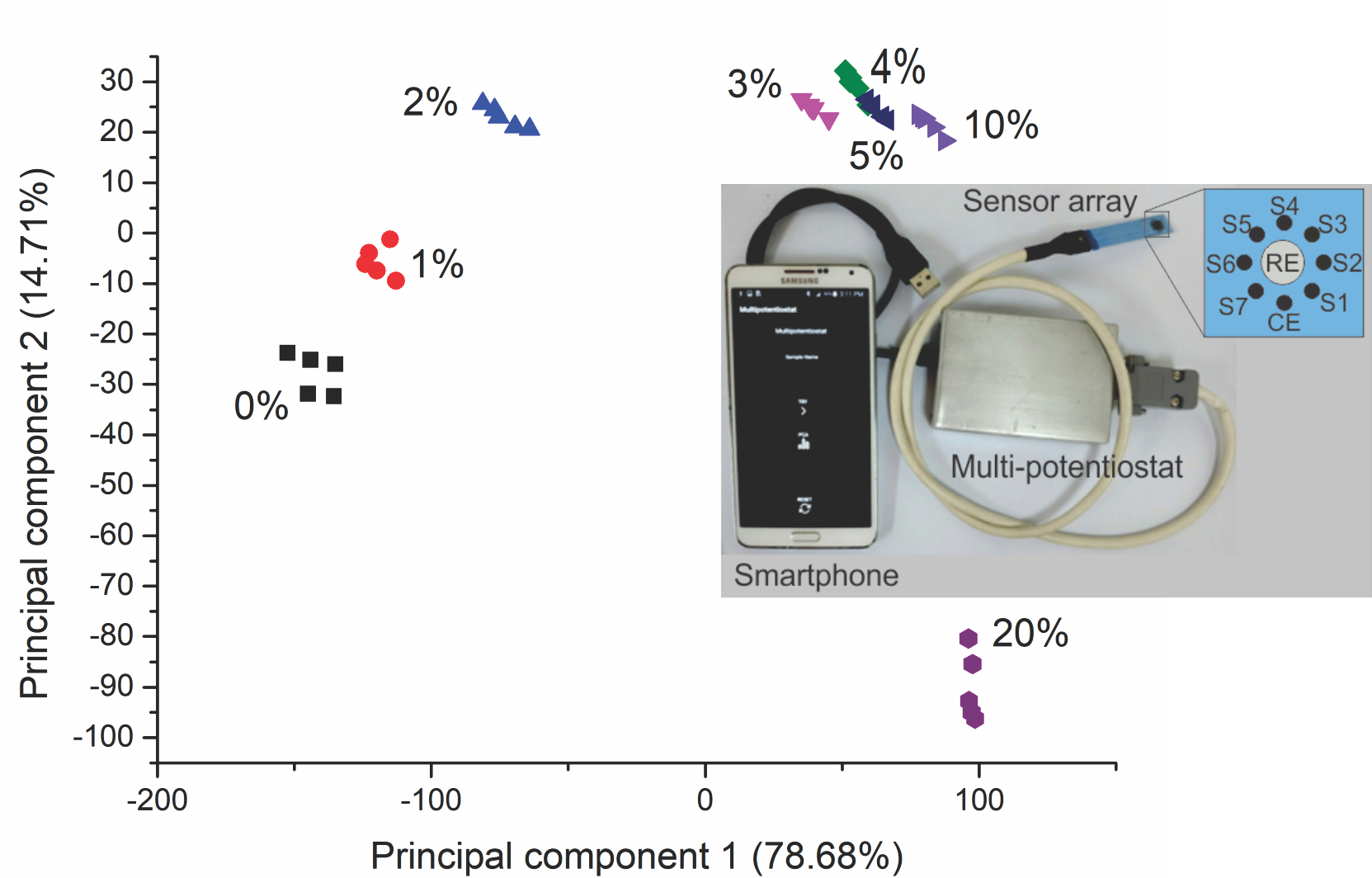
channels. Figure 1 depicts a smart electronic tongue. It can be seen the three
components that make it up as well as an expansion of the sensor array where
the reference electrode of Ag/AgCl of the array (RE), the counter electrode
(CE), and the array of seven sensors (S1…S7) can be observed.
Figure 1 Image of the smart electronic tongue formed by the
miniaturized sensor array, the portable electronic device, and a Smartphone
with an Android application for device control
To guarantee the proper functioning of
the sensors, their response to a raw milk sample was initially explored; all
the sensors were synthesized using a polymerization time of 70 s, with monomer
and doping agent concentrations of 0.20 M and 0.10 M, respectively.
Voltammetric signals were obtained without noise and with well-defined peaks
during the measurements. However, the sensors showed signals with some loss of
intensity, which could affect the measurements made with the smart electronic
tongue. As an example, Figure 2 demonstrates the response of the S7 sensor
(PPy/AQDS). It can be seen that the signal in the first recorded cycle had a
current intensity of 508.8 uA. After 50 consecutive cycles, the signal reached
a current intensity of 470.4 uA, representing a loss of intensity of 7.54%. For
the other sensors, the intensity losses recorded were 5.23%, 8.25%, 0.71%,
4.84%, 1.12%, 3.40% for PPy/SO4, PPy/DBS, PPy/SF, PPy/FCN, PPy/PC and PPy/TSA,
respectively. As previously described in other projects that
evaluated the response of polypyrrole sensors in various types of products, the
loss of intensity in the signals could be caused by the exit of the doping
agent from the polypyrrole matrix, which can be modulated by factors such as
the concentration of monomer and doping agent used in the synthesis solution
and the polymerization time (Arrieta et al., 2020; Foroughi et al., 2009).
Although the current losses do not
exceed 9%, which represents a low loss due to the sensors, in this case, no
more than 50 voltammetric cycles should be registered, and process optimization
of the sensor elaboration process was carried out to generate a more stable and
consistent signal over time. Therefore, monomer and doping agent concentration,
as well as polymerization time, were used to carry out the optimization process
by applying an experimental design in which the loss of intensity in the
signals, represented as a percentage of stability, was used as the output
variable.
Figure 2 Voltammetric signal of the PPy/AQDS sensor registered
in a raw milk sample
The results of the optimization
experiment design revealed that the analyzed factors influence the signals'
stability and the fact that there are interactions between them. Table 1
displays the results of the variance analysis performed with the PPy/TSA sensor.
The statistical significance of the factors and their interactions can be
appreciated, specifically polymerization time, the interaction between
polymerization time and concentration of the doping agent, and the interaction
between concentrations of monomer and doping agent used in the
electrodeposition process. In this case, it is possible to conclude that these
factors significantly impact the sensor signal's stability. These effects had P
values less than 0.05, indicating that they significantly differed from zero at
a 95 % confidence level. The most significant factor is the interaction between
the concentration of pyrrole and the concentration of the doping agent. It was
very important to determine an optimal value for these factors because they can
significantly interfere with the sensor array's performance when analyzing milk
samples.
The interactions and behavior of the
factors against stability were observed in the response surface graphs obtained
from the optimization study, as shown in Figure 3.
Response surface graphs and level curves were utilized to analyze the effects
of polymerization time, pyrrole concentration, and doping agent concentration
on signal stability (i.e., loss of signal intensity), which was used as the
response variable. This was done to obtain the optimal values of the levels for
each factor used in elaborating each sensor in the array.
It
was possible to determine that the dopant concentration presents optimum
stability at intermediate values close to 0.10 M. The concentration of pyrrole
for extreme values tends to increase the sensors' instability. In this sense,
the instability increases because of the rise in time and is offset by a high
or low dopant concentration. As for the time, the trend exhibits greater
instability using prolonged reaction times. It can also be seen that the lowest
areas of the response surface have the most suitable values to carry out the
elaboration of the sensors, which correspond to polymerization times of less
than 100 s, pyrrole concentration between 0.10 and 0.20 M, and a dopant
concentration between 0.05 and 0.10 M.
Table 1 Variance analysis of the
factors; polymerization time (t), the concentration of monomer [Py], and
concentration of doping agent [DA] obtained in the optimization process of the
PPy/TSA sensor
|
Source of variation |
Sum of Squares |
GI |
Mean Squares |
F |
P-Value |
|
A:Time
(t) |
169.341 |
1 |
169.341 |
6.24 |
0.0230 |
|
B:[Py] |
3.25976 |
1 |
3.25976 |
0.12 |
0.7331 |
|
C:[DA] |
5.95125 |
1 |
5.95125 |
0.22 |
0.6454 |
|
AA |
2.87042 |
1 |
2.87042 |
0.11 |
0.7489 |
|
AB |
52.0833 |
1 |
52.0833 |
1.92 |
0.1837 |
|
AC |
172.521 |
1 |
172.521 |
6.36 |
0.0219 |
|
BB |
4.335 |
1 |
4.335 |
0.16 |
0.6943 |
|
BC |
431.28 |
1 |
431.28 |
15.90 |
0.0010 |
|
CC |
73.2902 |
1 |
73.2902 |
2.70 |
0.1186 |
|
Error |
461.064 |
17 |
27.1214 |
|
|
|
Total
(corr.) |
1376.0 |
26 |
|
|
|
Based on the optimization results, the polymerization time
values and concentrations of monomer and pyrrole to be used in the elaboration
of each sensor were established. Table 2 illustrates the conditions used while
also showing the new values for the percentage of intensity loss obtained by
optimizing the processing parameters. It can be observed that the stability
improved remarkably, and the intensity losses did not exceed 3% in any of the
sensors, with very low values of 1% were obtained in the case of PPy/SO4 and
PPy/AQDS.
After
the sensor array was optimized, the measurements were carried out on the milk
samples to evaluate the ability of the smart electronic tongue to classify
samples adulterated with sucrose. The data matrix for the
principal component analysis was created from the signal registers. The
correlation circle generated by the principal components analysis is depicted
in Figure 4. Figure 4 shows that the data recorded in the signals generated by
the sensor array provide a large amount of information because most of the
points in the correlation circle are distributed at values close to 1 and -1 of
the quadrants; that is, the circle has a radius close to 1, indicating that the
sensor array provides a large amount of helpful information for sample
discrimination. In addition, it can be seen that the sensors do not provide
redundant information; this is evidenced by the fact that the areas of overlap
of points are scarce in the circle, inferring that
all of the sensors provide important information about the samples analyzed.
The variance or information captured in
the first two components was 93.39%, the first principal component with 78.68%,
and the second principal component with 14.71%. Figure 5 displays a score graph
demonstrating the discrimination of the samples of milk adulterated with
sucrose. It can be clearly seen that the samples with the same amount or
concentration of adulteration (i.e., sucrose) are clearly grouped together,
forming clusters corresponding to the various concentrations of adulterant studied.
Furthermore, the clusters are well differentiated from one another,
establishing clear discrimination of the samples analyzed.
Figure 3 Response surface of the most significant processing
factors for the PPy/TSA sensor
Table 2
Parameters used in the process of elaboration of the sensor array obtained from
the optimization process
|
Sensor |
Acronym |
Monomer/Doping
agent concentration [M] |
Polymerization
time (s) |
Loss of
intensity (%) |
|
S1 |
PPy/SO4 |
0.10/0.05 |
55 |
0.39 |
|
S2 |
PPy/DBS |
0.10/0.10 |
50 |
2.50 |
|
S3 |
PPy/SF |
0.10/0.05 |
65 |
3.00 |
|
S4 |
PPy/FCN |
0.10/0.10 |
60 |
1.98 |
|
S5 |
PPy/PC |
0.10/0.10 |
60 |
2.15 |
|
S6 |
PPy/TSA |
0.10/0.10 |
70 |
2.56 |
|
S7 |
PPy/ AQDS |
0.10/0.05 |
60 |
0.91 |
The unadulterated samples (0%) and the adulterated with 1%
are separated in the negative quadrant of the two components (quadrant -: -).
The sample adulterated with 2% is found in the negative quadrant of the first
component and positive in the second component (-: + quadrant), while the
groups of adulterated samples with 3%, 4%, 5%, and 10% are found in the
positive quadrants of both components (quadrant +: +). The sample with the
largest amount of adulterant (20%) is separated into the positive quadrant of
the first component and the negative quadrant of the second component (quadrant
+: -).
Figure 4 Correlation circle of the variables generated by the
sensor array registered in the principal component analysis
Although samples with 4% and 5% sucrose
concentrations touch each other, they do not overlap so that they can be
distinguished easily. Thus, each sample group is well discriminated, and the
smart electronic tongue could classify each sample based on its adulterant
content.
Figure 5 Correlation circle of the variables generated by the
sensor array registered in the principal component analysis
By using a smart electronic
tongue integrated with a polypyrrole sensor array, a portable measurement
device, and a smartphone made it possible to carry out measurements on
adulterated milk samples, obtaining well-defined signals. The elaboration
parameters of the sensors, such as polymerization time, monomer concentration,
and doping agent concentration, affect the stability of their voltammetric
signals against milk samples. Thereby, an optimization process had to be
carried out by applying an experimental design that allowed obtaining
well-defined signals with high stability. The optimal value of monomer
concentration was 0.10 M, the polymerization time was between 50 and 70 s, and
the doping agent concentration was between 0.05 and 0.10 M. The measurements on
the sucrose-adulterated samples allowed to register signals that contained
enough information to be able to differentiate them. Principal components 1 and
2 recorded a total of 93.39% variance, with 78.68% in principal component 1 and
14.71% in principal component 2. Thus, the result of the principal component
analysis demonstrated the discrimination capacity of the smart electronic
tongue on sucrose-adulterated samples.
The authors acknowledge the University of Sucre for the financial
support of this research.
| Filename | Description |
|---|---|
| R1-CE-5613-20220526233751.png | Figure 1 |
| R1-CE-5613-20220526233810.png | Figure 2 |
| R1-CE-5613-20220526233847.png | Figure 3 (R1) - New |
| R1-CE-5613-20220526233907.png | Figure 4 |
| R1-CE-5613-20220526233950.png | Figure 5 |
Alcañiz, M., Vivancos, J., Masot, R., Ibañez, J., Raga, M., Soto, J.,
Martínez-Máñez, R., 2012. Design of an Electronic System and its Application to
Electronic Tongues Using Variable Amplitude Pulse Voltammetry and Impedance
Spectroscopy. Journal of Food Engineering, Volume 111(1), pp. 122–128
Arrieta, A.A., Fuentes, O., 2016. Portable Electronic Tongue for the
Analysis of Raw Milk Based on PSoC (Programmable System on Chip) and Android Technology.
Ingeniare: Journal Chilean engineering, Volume 24(3), pp. 445–453
Arrieta, A.A., Fuentes, O., Palencia, M., 2015. Android and PSoC
Technology Applied to Electronic Tongue Development. Journal of Applied
Sciences, Engineering and Technology, Volume 10(7), pp. 782–788
Arrieta, A.A., Nuñez, Y.E., Mendoza, J.M., 2020.
Mini-electronic Tongue Used to Discriminate between Coffee Samples of Different
Geographical Origin. International Journal of Technology, Volume 11(2),
pp. 288–298
Arrieta, A.A., Palencia, M., Fuentes, O., 2018. Miniaturised Multi-Channel
System of Electrochemical Measurements for an Electronic Tongue for Milk
Samples. Maejo International Journal of Science and Technology, Volume
12(1), pp. 28–35
Azad, T., Ahmed, S., 2016. Common Milk Adulteration and Their Detection
Techniques. International Journal of Food Contamination, Volume 3(1),
pp. 1–9
Borges, T.H., Peres, A.M., Dias, L.G., Seiquer, I., Pereira, J.A., 2018.
Application of a Potentiometric Electronic Tongue for Assessing Phenolic and
Volatile Profiles of Arbequina Extra Virgin Olive Oils. LWT, Volume 93,
pp. 150–157
Dos-Santos, P.M., Costa, L.F., Pereira-Filho, E.R., 2012. Study of
Calcium and Sodium Behavior to Identify Milk Adulteration Using Flame Atomic
Absorption Spectrometry. Food and Nutrition Sciences, Volume 3(9), pp.
1228–1232
Facure, M.H.M., Schneider, R., Dos-Santos, D., Correa, D.S., 2020.
Impedimetric Electronic Tongue Based on Molybdenum Disulfide and Graphene Oxide
for Monitoring Antibiotics in Liquid Media. Talanta, Volume 217, pp. 1–9
Foroughi, J., Spinks, G.M., Wallace, G.G., 2009. Effect of Synthesis
Conditions on the Properties of Wet Spun Polypyrrole Fibers. Synthetic
Metals, Volume 159(17), pp. 1837–1843
Guetouache, M., Guessas, B., Medjekal, S., 2014. Composition and
Nutritional Value of Raw Milk. Issues in Biological Sciences and
Pharmaceutical Research, Volume 2(10), pp. 115–122
Hayat, M., Saepudin, E., Einaga, Y., Ivandini, T.A., 2019. CdS Nanoparticle-based
Biosensor Development for Aflatoxin Determination. International Journal of
Technology. Volume 10(4), pp. 787–797
Katseli, V., Thomaidis, N., Economou, A., Kokkinos, C., 2020. Miniature
3D-Printed Integrated Electrochemical Cell for Trace Voltammetric Hg(II)
Determination. Sensors and Actuators B: Chemical, Volume 308, p. 127715
Khalil, M., Liu, N., Lee, R.L., 2018. Super-Nernstian Potentiometric pH
Sensor Based on the Electrodeposition of Iridium Oxide Nanoparticles. International
Journal of Technology. Volume 9(3), pp. 446–454
Lipkowitz, J.B., Ross, C.F., Diako, C., Smith, D.M., 2018. Discriminating
Aging and Protein-To-Fat Ratio in Cheddar Cheese using Sensory Analysis and
Potentiometric Electronic Tongue. Journal of Dairy Science, Volume
101(3), pp. 1990–2004
Medina-Plaza, C., García-Hernandez, C., De Saja, J.A.,
Fernández-Escudero, J.A., Barajas, E., Medrano, G., García-Cabezón, C.,
Martin-Pedrosa, F., Rodriguez-Mendez, M.L., 2015. The Advantages of Disposable
Screen-Printed Biosensors in a Bioelectronic Tongue for the Analysis of Grapes.
LWT-Food Science and Technology, Volume 62(2), pp. 940–947
Oroian, M., Ropciuc, S., 2019. Romanian Honey Authentication using a
Voltammetric Electronic Tongue. Correlation of Voltammetric Data with Physico-Chemical
Parameters and Phenolic Compounds. Computers and Electronics in Agriculture,
Volume 157, pp. 371–379
Song, X., She, S., Xin, M., Chen, L., Li, Y., Vander-Heyden, Y., Rogers,
K.M., Chen, L., 2020. Detection of Adulteration in Chinese Monofloral Honey
Using 1H Nuclear Magnetic Resonance and Chemometrics. Journal of
Food Composition and Analysis, Volume 86, p. 103390
Vlasov, Y., Legin, A., Rudnitskaya, A., Di-Natale, C., D'Amico, A.,
2005. Nonspecific Sensor Arrays ("Electronic Tongue") for Chemical
Analysis of Liquids (IUPAC Technical Report). Pure Applied Chemistry,
Volume 77(11), pp. 1965–1983
Wang, Y., Wang, L., Li, Z., 2020. Dynamic Analysis of China’s Imported
Raw Milk Powder Consumption. Sustainability, Volume 12(4), pp. 1542–1557
Wei, Z., Wang, J., Zhang, X., 2013. Monitoring of Quality and Storage
Time of Unsealed Pasteurized Milk by the Voltammetric Electronic Tongue. Electrochimica
Acta, Volume 88, pp. 231–239
Winquist, F., Bjorklund, R., Krantz-Rülcker, C., Lundström, I.,
Östergren, K., Skoglund, T., 2005. An Electronic Tongue in the Dairy Industry. Sensors
and Actuators B: Chemical, Volume 111, pp. 299–304
Witkowska, E., Kubota, L., 2016. Integrated, Paper-Based Potentiometric
Electronic Tongue for the Analysis of Beer and Wine. Analytica Chimica Acta,
Volume 918, pp. 60–68
Yang, Y., Huisman, W., Hettinga, K.A., Zhang, L., Van-Ruth, S.M., 2020.
The Chinese Milk Supply Chain: A fraud perspective. Food Control, Volume
113, pp. 1–10
Zabadaj, M., Szuplewska, A., Kalinowska, D., Chudy, M.,
Ciosek-Skibi?ska, P., 2018. Studying Pharmacodynamic Effects in Cell Cultures
by Chemical Fingerprinting?SIA Electronic Tongue Versus 2D Fluorescence Soft
Sensor. Sensors and Actuators B: Chemical, Volume 272, pp. 264–273
