Impedantiometric behavior of solid biopolymer electrolyte elaborated from cassava starch synthesized in different pH
Published at : 07 Oct 2022
Volume : IJtech
Vol 13, No 4 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i4.5612
Arrieta, A., Barrera, I., Mendoza, J., 2022. Impedantiometric behavior of solid biopolymer electrolyte elaborated from cassava starch synthesized in different pH. International Journal of Technology. Volume 13(4), pp. 912-920
| Alvaro Arrieta | Department of Biology and Chemistry, University of Sucre, Road 28 No. 5-267 Red Door neighborhood, Sincelejo, Colombia, Postal Code 700008 |
| Isora Barrera | Mercedes Abrego Educational Institution, Diag. 6-119, Tv. 1 #6-1, Monteria, Colombia, Postal Code 230004 |
| Jorge Mendoza | Department of Mechanical Engineering, University of Córdoba, Monteria, Colombia, Postal Code 230002 |
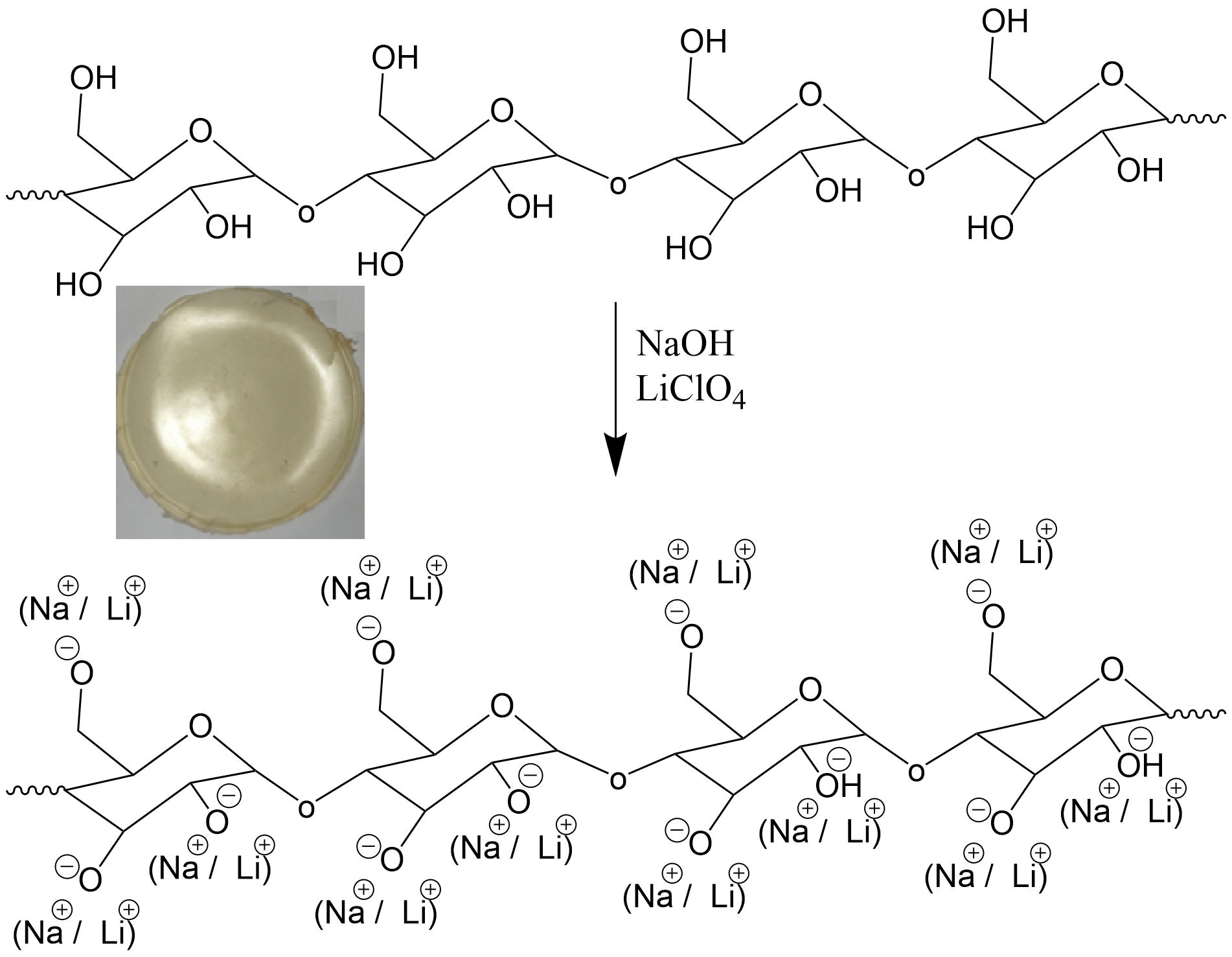
This
paper approaches the study of pH’s effect on elaborate films of solid
biopolymer electrolyte from cassava starch and its impedantiometric response.
The films of solid biopolymer electrolyte were elaborated by thermochemical
synthesis while varying the pH (2, 4, 5, 7, 9, 10, and 12). Starch was
extracted from cassava tubers by a traditional method (disintegrated, washed,
decanted, filtered, and dried). Solid biopolymer electrolyte films were processed
by thermochemical synthesis by adding
plasticizers (glycerol, glutaraldehyde, and polyethylene glycol) and lithium
salt (lithium perchlorate). The impedance behavior was studied using the
electrochemical impedance spectroscopy technique. The
Nyquist and Bode’s diagrams registered presented a similar trend in all the
films; therefore, they were described by the same equivalent circuit model.
However, the equivalent circuit components presented different values in each
case. The conductivity and capacitance showed a quadratic polynomial tendency
in relation to the pH, obtaining the highest conductivity in the films
elaborated at acidic pH and the highest capacitance in the films elaborated at
basic pH. The degree of basicity or
acids allowed conductivity to be modulated or capacitance of the solid
biopolymer electrolyte as required. It could be concluded that the production
pH has a marked effect on impedantiometric behavior of films of solid
biopolymer electrolyte from cassava starch, which may be useful to modulate the
electrochemical properties of this type of material in future Applications.
Cassava; Electrochemical impedance; pH; Solid biopolymer electrolyte; Starch
Within the wide range of materials available, polymers
are undoubtedly the most used in the development of an ample variety of
utensils and devices for everyday use. Polymers are very popular due to their
excellent mechanical properties, high resistance to attack by organic and
inorganic solvents, and corrosion resistance, among others. The global use of
polymers is so great that they have now become an environmental problem
worldwide, as they constitute one of the main sources of pollution (Zhong et al., 2019; Dwivedi et al., 2019; Emi-Cassola et
al., 2019).
Polymers
are considered highly polluting materials (Dwivedi
et al., 2019; Emi-Cassola et al., 2019; Shonnard et al., 2019). This contingency
has carried numerous research centers worldwide to seek alternatives to
replace synthetic polymers (petrochemical origin) worldwide to seek alternatives to replace synthetic polymers
(petrochemical origin) with polymers elaborated from biological sources
(biopolymers). Biopolymers can have similar properties to synthetic polymers,
so they can be used in a wide range of applications and are also friendly to
the environment, due to their good biodegradability, low production cost and
originating from renewable sources (Mohamed et al.,
2018).
Due to their high technological and industrial
potential, the most used and studied biopolymers are cellulose, alginate,
starch, chitosan, among others (Rochardjo et al.,
2021; Imani et al., 2022; Jyothi et al., 2019). However, the low
electrical conductivity of conventional polymers and biopolymers has limited
their applications in various new electronic devices. In recent decades, the
development of polymers capable of conducting electric current (i.e.,
conducting polymers) has opened the possibility of applying such materials in
applications as diverse as smart windows, solar cells, sensors, artificial
muscles, capacitors, electrochemical accumulators, electroluminescent diodes
(LEDs), touch panels, among others (Cichosz et al.,
2018; Itik et al., 2015; AL-Barani et al., 2019).
Conducting polymers are divided into two groups; the
intrinsic conducting polymers (ICP), which can conduct electric current across
the chains with conjugated bonds and charges generated by oxidation or
reduction (Awuzie, 2017; Chen et al., 2021).
On the other hand, the ionic conducting polymers, which have fixed charges in
their chains and mobile ions that produce the electroneutrality in the matrix,
in this type of materials the movement of mobile ions gives the conduction, so
they are known as polymeric solid electrolytes (Aziz
et al., 2018; Angell, 2019). Intrinsic and ionic conducting polymers are
mostly synthetic and therefore cause
environmental problems typical of polymers from a petrochemical origin.
Few works report the synthesis of biopolymers capable
of conducting electric current (Arrieta et al.,
2011; Mobarak et al., 2015). Recently, it has been reported the use of
cassava starch to generate a conducting biopolymer with the use of plasticizers
and lithium salt (Arrieta et al., 2011).
This biopolymer has been studied as a solid electrolyte for application in an
artificial muscle and as an electrochemical accumulator (Núñez et al., 2016; Arrieta et al., 2019a). However, not many
studies have been conducted about the effect of synthesized pH on the electrochemical
properties of this biopolymeric solid electrolyte. The effect of the synthesized pH on voltametric response
and mechanical properties of this type of biopolymer material was reported
recently, showing that factors such as redox potentials (oxidation/reduction),
crystallinity, voltametric stability, modulus of elasticity and electrical
conductivity can be affected. (Arrieta et
al., 2019b; Arrieta et al., 2018). In this work, the study of a synthesized
pH effect (pH values; 2, 4, 5,
7, 9, 10, and 12) on a conducting
biopolymer elaborated from cassava starch about its impedance behavior is
presented.
Films of solid biopolymer electrolytes can be
elaborated using cassava starch. The films were stable against handling;
however, the films synthesized at pH 2 values were brittle and broke during
handling. Therefore, the films presented stability when they were elaborated at
a pH higher than 4. The impedaciometric behavior of the films showed a similar
trend in all cases, is defined by a similar equivalent circuit model. However,
the values of the equivalent circuit
components were different in each case. The pH used during the synthesis
process affects the electrochemical properties of cassava starch solid
biopolymer electrolyte films. The conductivity in the films presented a
polynomial relationship (degree 2).
The films elaborated at low pH registered a higher conductivity. On the other
hand, the capacitance of the films showed a behavior opposite to the
conductivity, being higher in the films synthesized at a more basic pH. This
behavior could be due to the variation in the crystallinity of the biopolymeric
films, which is influenced by the synthesized pH. The conduction mechanisms in solid
biopolymer electrolyte films are not affected by pH; however, their capacitive
and electrical conduction capacity are altered. In this way, it was determined that the variation in the
synthesized pH allows to modulate the properties of conductivity or capacitance
according to the application to which the solid biopolymer electrolyte is
intended. In future works, the application of this material in smart fertilizer
release systems will be studied.
The authors acknowledgment to The Ministry of Science, Technology,
and Innovation (Minciencias) - Colombia for the financial support provided to
the project code BPIN 2020000100027 through resources from the General System
of Royalties (SGR).
| Filename | Description |
|---|---|
| R1-CE-5612-20220427233748.png | Figure 1 |
| R1-CE-5612-20220427233813.png | Figure 2 |
| R1-CE-5612-20220427233839.png | Figure 3 |
| R1-CE-5612-20220427233901.png | Figure 4 |
| R1-CE-5612-20220427233924.png | Figure 5 |
| R1-CE-5612-20220427233951.png | Figure 6 |
| R1-CE-5612-20220427234008.png | Figure 7 |
| R1-CE-5612-20220427234027.png | Figure 8 |
AL-Baradi, A.M., Al-Shehri, W.A., Badawi,
A., Almalki, A.S.A., Merazga, A., 2019. A Study of the Nanostructure and
Efficiency of Solid-State Dye-Sensitized Solar Cells Based on a Conducting
Polymer. Heliyon, Volume 5(4), p. e01472
Angell, C.A., 2019. Concepts and Conflicts in
Polymer Electrolytes: The Search for Ion Mobility. Electrochimica Acta,
Volume 313(1), pp. 205–210
Arrieta, A.A., Mendoza, J.M., Arrieta,
P.L., 2019a. Evaluation of Elaboration Parameters of a Solid Biopolymer
Electrolyte of Cassava Starch on Their Performance in an Electrochemical
Accumulator. Revista Mexicana de Ingeniería Química,
Volume 18(3), pp. 1203–1210
Arrieta, A., Garcia, C., Combatt, E., 2019b.
Effect of Elaboration ph on the Electroactivity of
Cassava Starch Solid Biopolymer Electrolyte Films. Rasayan Journal of
Chemistry, Volume 12(4), pp. 1766–1773
Arrieta, A.A., Gañán, P.F., Márquez, S.E.,
Zuluaga, R., 2011. Electrically Conductive Bioplastics from Cassava Starch. Journal
of the Brazilian Chemical Society, Volume 22(6), pp. 1170–1176
Arrieta, A., Montoya, M., Palencia, M.,
2018. Electrochemical Study of Cassava Starch Conductive
Biopolymers Synthesized at Different pH. Advance Journal of Food Science and
Technology, Volume 15, pp. 148–151
Awuzie, C.I., 2017. Conducting Polymers. Materials
Today: Proceedings, Volume 4(4), pp. 5721–5726
Aziz, S.B., Woo, T.J., Kadir, M.F.Z.,
Ahmed, H.M., 2018. A Conceptual Review on Polymer Electrolytes and Ion
Transport Models. Journal of Science: Advanced Materials and Devices,
Volume 3(1), pp. 1–17
Cichosz, S., Masek, A., Zaborski, M., 2018.
Polymer-based Sensors: A Review. Polymer Testing. Volume 67, pp. 342–348
Chen, Z., Villani, E., Inagi, S., 2021.
Recent Progress in Bipolar Electropolymerization Methods Toward One-Dimensional
Conducting Polymer Structures. Current Opinion in Electrochemistry,
Volume 28, p. 10070
Dwivedi, P., Mishra, P.K., Mondal, M.K.,
Srivastava, N., 2019. Non-biodegradable Polymeric Waste Pyrolysis for Energy
Recovery. Heliyon, Volume 5(8), pp. 1–15
Itik, M., Sahin, E., Ayas, M.S., 2015.
Fractional Order Control of Conducting Polymer Artificial Muscles. Expert
Systems with Applications, Volume 42(21), pp. 8212–8220
Jyothi, S., Subba Rao, Y.V., Samuel
Ratnakumar, P.S., 2019. Natural Product as Corrosion Inhibitors in Various
Corrosive Media: A Review. Rasayan Journal Chemical, Volume 12(2), pp.
537–544
Imani,
N.A.C., Kusumastuti, Y., Petrus, H.T.B.M., Timotius, D., Putri, N.R.E.,
Kobayashi, M., 2022. Preparation, Characterization, and Release Study of
Nanosilica/Chitosan Composite Films. International Journal of Technology,
Volume 13(2), pp. 444–453
Khanmirzaei, M.H., Ramesh, S., 2013. Ionic Transport
and FTIR Properties of Lithium Iodide Doped Biodegradable Rice Starch Based
Polymer Electrolytes. International Journal of Electrochemical Science,
Volume 8, pp. 9977–9991
Li, G., Li, Z., Zhang, P., Zhang, H., Wu,
Y., 2008. Research on a Gel Polymer Electrolyte for Li-Ion Batteries. Pure
and Applied Chemistry, Volume 80, pp. 2553–2563
Mobarak, N.N., Jumaah, F.N., Ghani, M.A.,
Abdullah, M.P., Ahmad, A., 2015. Carboxymethyl Carrageenan Based Biopolymer
Electrolytes. Electrochimica Acta, Volume 175, pp. 224–231
Mohamed,
M.H., Ajaero, C., McMartin, D.W., Peru, K.M., Friesen, V., Simair, M., Headley,
J.V., Wilson, L., 2018. Solubilized Chitosan Biopolymers for Sequestration of
Organic Acids in Aquatic Environments after Biodegradation in a Constructed
Wetland Treatment System. International Journal of Technology, Volume
9(6), pp. 1140–1150
Núñez,
Y.E., Arrieta, A.A., Segura, J.A., Bertel, S.D., 2016. Synthesis of an Air-Working
Trilayer Artificial Muscle Using a Conductive Cassava Starch Biofilm (manihot
esculenta, cranz) and Polypyrrole (PPy). Journal of Physics: Conference
Series, Volume 687, pp. 1–4
Rochardjo,
H.S., Fatkhurrohman, Kusumaatmaja, A., Yudhanto, F., 2021. Fabrication of
Nanofiltration Membrane Based on Polyvinyl Alcohol Nanofibers Reinforced with
Cellulose Nanocrystal using Electrospinning Techniques. International Journal
of Technology, Volume 12(2), pp. 329–338
Shonnard, D., Tipaldo, E., Thompson, V.,
Pearce, J., Caneba, G., Handler, R., 2019. Systems Analysis for PET and Olefin Polymers
in a Circular Economy. Procedia CIRP, Volume, 80, pp. 602–606
Zhang, L., Shen, H., Luo, Y., 2010. Study
on the Electric Conduction Properties of Fresh and Frozen-Thawed Grass Carp (Ctenopharyngodon
Idellus) and Tilapia (Oreochromis niloticus). Journal Food Science and
Technology, Volume 45, pp. 2560–2564
Zhong, C., Zhao, H., Cao, H., Huang, Q., 2019.
Polymerization of Micropollutants in Natural Aquatic Environments: A Review. Science
of The Total Environment, Volume 693, pp. 1–21
