Atmospheric Leaching Behavior and Kinetics of Nickel and Cobalt from Halmahera Limonite Ore
Corresponding email: bayupetrus@ugm.ac.id
Published at : 10 Jul 2024
Volume : IJtech
Vol 15, No 4 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i4.5607
Astuti, W., Avista, D., Prihutami, P., Wanta, K.C., Prakosa, A., Anggara, F., Petrus, H.T.B.M., 2024. Atmospheric Leaching Behavior and Kinetics of Nickel and Cobalt from Halmahera Limonite Ore. International Journal of Technology. Volume 15(4), pp. 824-833
| Widi Astuti | Research Center for Mining Technology, National Research and Innovation Agency (BRIN), Jl. Ir. Sutami Km. 15, Tanjung Bintang, Lampung Selatan, Lampung, 35361, Indonesia |
| Dira Avista | Research Center for Mining Technology, National Research and Innovation Agency (BRIN), Jl. Ir. Sutami Km. 15, Tanjung Bintang, Lampung Selatan, Lampung, 35361, Indonesia |
| Pramesti Prihutami | Sustainable Resources Engineering, Hokkaido University, Sapporo, Hokkaido, 060-8628, Japan |
| Kevin Cleary Wanta | Department of Chemical Engineering, Faculty of Industrial Technology, Parahyangan Catholic University, Jl. Ciumbuleuit 94, Bandung, 40141, Indonesia |
| Agus Prakosa | Research Center for Mining Technology, National Research and Innovation Agency (BRIN), Jl. Ir. Sutami Km. 15, Tanjung Bintang, Lampung Selatan, Lampung, 35361, Indonesia |
| Ferian Anggara | 1. Department of Geological Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika 2, Bulaksumur, Yogyakarta, 55281, Indonesia 2. Faculty of Engineering, Unconventional Geo-Resourc |
| Himawan Tri Bayu Murti Petrus | 1. Faculty of Engineering, Unconventional Geo-Resources Research Group, Universitas Gadjah Mada, Jl. Grafika, No. 2, Yogyakarta, 55281, Indonesia 2. Department of Chemical Engineering, Faculty of Eng |
Halmahera Island in Indonesia holds great potential
for nickel laterite due to its geological setting. As the product of chemical
weathering, nickel laterite can be divided into saprolite and limonite. Despite
containing invaluable elements like nickel and cobalt, limonite ore is seldom
used in metal production. This study evaluates these two elements’ leaching
behavior and kinetics from the Halmahera deposit. Limonite ore was leached
using 50 mL of 0.5 M sulfuric acid at an S/L ratio of 10% and 20%. The leaching
process was conducted at 30°C, 50°C, and 80°C. The experimental results showed
that the highest recovery for cobalt and nickel was 100% in the adequate
presence of H+ ions. The result shows that sulfuric acid is more
selective to cobalt than a nickel. The shrinking core ash diffusion model
represents the leaching kinetics of cobalt, while nickel recovery follows the
Kröger-Ziegler kinetics model.
Cobalt; Kinetics; Leaching; Limonite; Nickel
Kinetic studies are the most important part of the design process of a processing unit. By studying the kinetics of this process, the physical phenomena in a deep process are known. Thus, the process of designing and scaling up a unit can be carried out according to the most real phenomena, and design errors can be minimized. Matching the most appropriate kinetics model from a shrinking core model (Mirwan et al., 2017) to the newest product diffusion developed model (Wanta et al., 2022; Prihutami et al., 2022), will be the objective of this study. Thus, the kinetics parameter needed for scale-up can be generated for sulfuric acid leaching on Halmahera limonite at atmospheric conditions.
2.1. Materials
The limonitic laterite ore used in this
study was from a mining area on Halmahera Island, Indonesia. A representative
sample was obtained by coning and quartering. The dried sample was crushed,
ground, ball milled, and sieved until the average size of the sample was < 74 The chemical components of the selected representative sample were
determined using X-ray Fluorescence (XRF, Epsilon 3XLE PANalytical). X-ray Diffraction (XRD, X’Pert
3 Powder from PANalytical, Netherlands) was also used to identify the
crystalline materials in limonite ore. The morphology and elemental map of the
sample were analyzed using FE-SEM/EDS Thermo Scientific Quattro S. Pregnant
leached solution in this study was prepared by diluting pro analytical sulfuric
acid (CAS RN of 7664-93-9, Merck, Germany) in aquadest.
2.2. Menthods
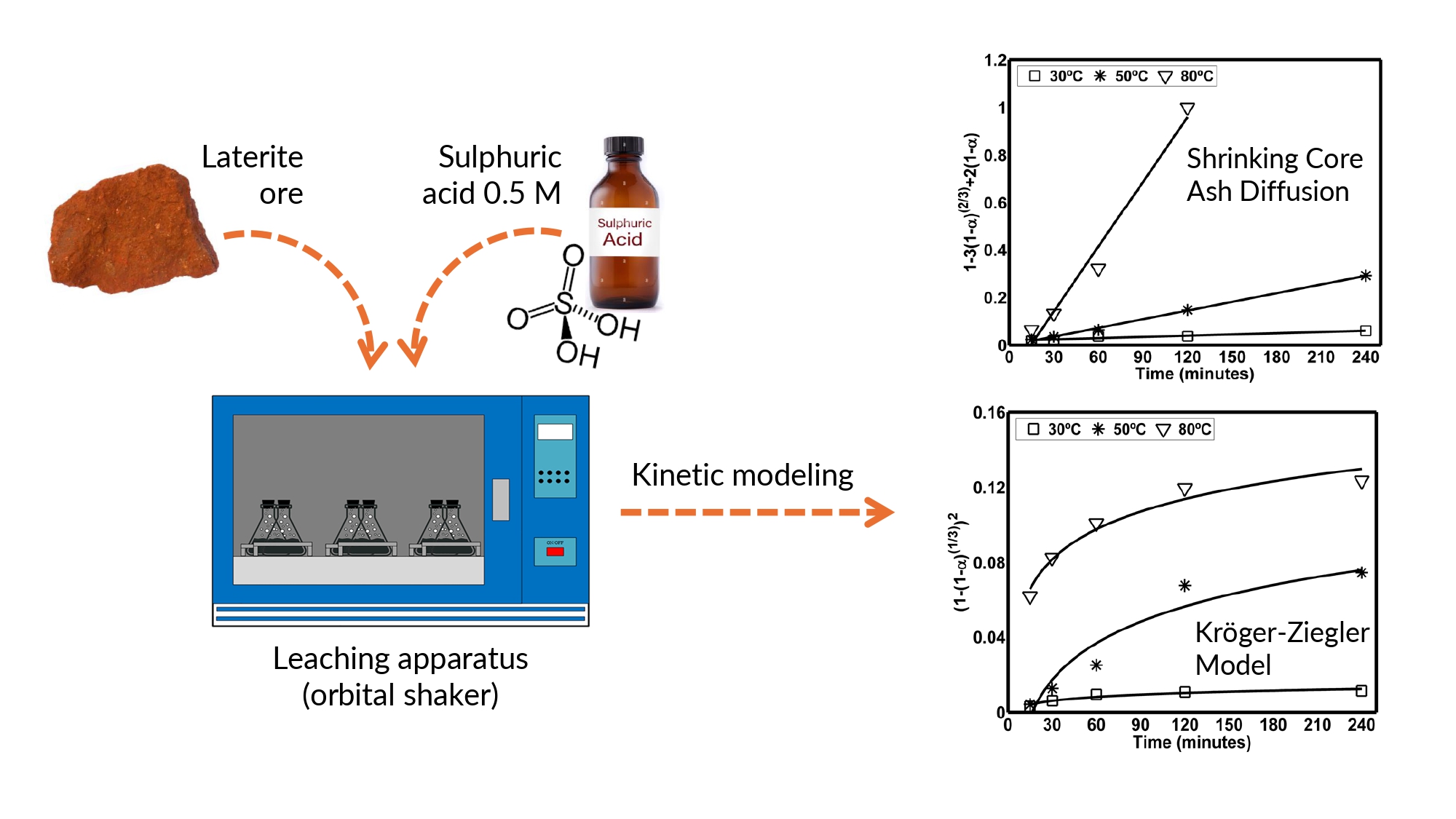
Halmahera limonite ore was put into an Erlenmeyer, and 50 mL of 0.5 M sulfuric acid was added. The atmospheric leaching was done for 4 hours in an orbital shaker, as shown in Figure 1. The shaker speed was kept constant at 200 rpm, while the S/L ratio and leaching temperature varied from 30 to 80°C. The concentration of leached nickel and cobalt at any designated time (15 minutes, 30 minutes, an hour, 2 hours, and 4 hours) was determined using ICP-Optical Emission Spectrometer PQ 9000 from Analytik Jena AG, Germany.
Figure 1 The experimental apparatus for
nickel and cobalt leaching: (1) orbital shaker; (2) Erlenmeyer flask
where C is the element concentration in
the liquid sample (g/mL), V is the volume of sulfuric acid solution (50 mL), X
is the element concentration in ore, and m is the mass of the limonite ore (g).
kK is the leaching rate
constant for the Kröger-Ziegler model, t is the leaching time (min), is the
recovery value of either nickel or cobalt, and kD is the leaching rate constant
for the ash diffusion controls model.
3.1. Ore Characterization
Figure 3 Elemental Mapping of Limonite Ore: Cr (dark blue); O (light green); Mg
(blue); Si (tosca); Fe (purple); Ni (yellow); Al (orange); Mn (green)
XRF analysis shows that
Halmahera limonite ore contains 0.12% of cobalt. This element is mainly carried
by Mn-oxyhydroxides, like asbolane and lithiophorite-asbolane (Sagapoa, Imai, and Watanabe, 2011; Tupaz et al.,
2020). These minerals have poor crystallinity and thus hardly appear on
diffractograms (Burlet and Vanbrabant, 2015; Maci?g
et al., 2019).
However, the XRF result indicates the presence of manganese
oxides, which undergo coprecipitation with cobalt and other elements, such as
Ni (1.73 wt%), Co (0.12 wt%), Fe (34.12 wt%), Mn (0.99 wt%), Si (3.46 wt%), and
Al (1.46 wt%). Aside from Mn-oxyhydroxides, cobalt is also hosted in lizardite
and some in goethite (Tang and Valix, 2004;
Sufriadin et al., 2020). FE-SEM/EDS analysis was also used to
confirm the XRF data. FE-SEM/EDS result indicates the presence of Ni (1.72
wt%), Fe (26.17 wt%), Mg (10.80 wt%), Al (1.39 wt%), Cr (0.37 wt%), Mn (0.47
wt%), and Si (9.86 wt%) (Astuti et al.,
2021).
3.2. Nickel and Cobalt
Leaching
The leaching process of nickel and cobalt from
Halmahera limonite ore by sulfuric acid was done at 30°C, 50°C, and 80°C. Along
with the increase in temperature, the recovery of both elements also increases.
Higher temperature provides greater kinetic energy, which allows rapid
molecular movement. The fast movement accelerates molecular diffusion and
surface reaction. This trend is the same at an S/L ratio of 10% (Figure 4) and
20% (Figure 5).
Unlike temperature, the S/L ratio is inversely
proportional to nickel and cobalt recovery. At 10% of the S/L ratio, sulfuric
acid can recover 100% of cobalt after an hour. Meanwhile, the same value is
achieved by nickel after 4 hours at 80°C. Complete recoveries are possible
since there are adequate hydrogen ions (H+) to leach both elements
from their carrier. However, a distinct case is found using a higher S/L ratio.
Loading more solids into the system raises the number of leachable materials.
However, the amount of H+ is insufficient to recover all available
nickel and cobalt. At this point, leached solution selectively reacts with the
host mineral that is easier to dissolve.
Figure 5 Nickel and Cobalt Recovery using an S/L Ratio of 20% at a) 30°C, b)
50°C, and c) 80°C
Using 20% of the S/L ratio at any temperature, the leaching process shows that cobalt recovery increases proportionally with time. Meanwhile, the recovery of nickel initially surges but slows down after some time. At 80°C, the leaching of nickel does not surpass 72.72% even after 4 hours, while cobalt achieves complete recovery only after 2 hours. This phenomenon indicates that sulfuric acid dissolves cobalt-rich minerals, like lizardite and manganese oxide, more quickly than nickel-rich minerals (Astuti et al., 2016). As goethite is the main component in Halmahera limonite ore, a great amount of acid will be needed to dissolve said mineral and recover nickel, according to Equations 5 and 6 (Senanayake et al., 2011).
3.3. Leaching Kinetics
Knowing the leaching kinetics of elements will
be useful for the industrialization process. In this study, leaching kinetics
are evaluated using a high S/L ratio. It is assumed that the rate-controlling
step would be diffusional mass transfer through an ash layer. According to the
data processing result, the recovery of cobalt and nickel from Halmahera
limonite ore follows this assumption. However, these two elements have a
slightly different phenomena during the leaching process.
Figure 6 (a) Shrinking Core Ash Diffusion Model for Cobalt
Leaching at Various Temperatures, and (b) Kröger-Ziegler Model for
Nickel Leaching at Various Temperature
One thing that differentiates the shrinking
core model from the Kröger-Ziegler model is that its effective diffusion
coefficient is assumed to be constant (Levenspiel,
1999). In the Kröger-Ziegler model, the effective diffusion coefficient
is inversely correlated with leaching time (Prihutami
et al., 2021). In a condition that lacks acid, the leaching of
limonite leaves behind unreacted goethite (Sahu, Kavuri, and Kundu, 2011). This ash layer thickens with time, hinders
molecular diffusion, and lowers nickel’s diffusion coefficient, which mainly
exists in goethite. This phenomenon does not happen to cobalt as it is mainly
hosted in much more leachable minerals.
In
this work, temperature positively affects nickel and cobalt recovery, unlike
the S/L ratio. At a high S/L ratio, the system lacks H+ activity,
and sulfuric acid selectively leaches more cobalt than a nickel. Due to the
formation of an ash layer (unreacted goethite), the leaching kinetics is
controlled by molecular diffusion through the ash layer. However, the host
mineral greatly determines the leaching phenomenon. Thus, cobalt and nickel
have two different kinetics models. The shrinking core model suits cobalt
leaching well, while the Kröger-Ziegler model best describes the nickel
leaching phenomenon. Furthermore, in future work, the mathematical models will
be explored further so that kinetic information which describes the actual
physical phenomena of this leaching process can be obtained. The model can then
be applied to the design extractor.
Highly appreciation
to BRIN for the analytical instruments provided as well as RIIM (Riset dan
Inovasi Indonesia Maju) LPDP for the financial support with contract B-807/II.7.5/FR/6/2022 and B-6952/III.10/KS.00.00/6/2022.
Aarabi-Karasgani, M., Rashchi, F., Mostoufi, N.,
Vahidi, E., 2010. Leaching of Vanadium from Linz–Donawitz (LD) Converter Slag using Sulfuric Acid. Hydrometallurgy,
Volume 102(1-4), pp. 14–21
Ahmed, I.M., Nayl, A.A., Daoud, J.A., 2016.
Leaching and Recovery of Zinc and Copper from Brass Slag by Sulfuric Acid. Journal of Saudi Chemical Society,
Volume 20, pp. S280–S285
Astuti, W., Hirajima, T., Sasaki, K., Okibe, N.,
2016. Comparison of Atmospheric Citric Acid Leaching Kinetics of Nickel from Different Indonesian Saprolitic Ores. Hydrometallurgy, Volume 161, pp. 138–151
Astuti, W., Mufakhir, F.R., Nurjaman, F.,
Sumardi, S., Herlina, U., Bahfie, F., Petrus, H.T.B.M., 2021. Pengaruh Karakteristik
Bijih pada Ekstraksi Nikel dari Bijih Limonit Indonesia Menggunakan Pelindian
Atmosferik (The Effect of Ore
Characteristics on Nickel Extraction from Indonesian Limonite Ore Using
Atmospheric Leaching). Metal Indonesia, Volume
43(1), pp. 9–16
Botelho Junior, A.B., Dreisinger, D.B., Espinosa,
D.C.R., Tenório, J.A.S., 2018. Pre-reducing Process Kinetics to Recover Metals from Nickel Leach Waste using Chelating Resins. International Journal of Chemical Engineering, Volume 2018, pp. 1–7
Burlet, C., Vanbrabant, Y., 2015. Study of the Spectro-Chemical Signatures of Cobalt-Manganese Layered Oxides (Asbolane-Lithiophorite and Their Intermediates) by
Raman Spectroscopy. Journal of Raman Spectroscopy, Volume 46(10), pp. 941–952
Butt, C.R.M., Cluzel, D., 2013. Nickel Laterite Ore Deposits: Weathered Serpentinites. Elements, Volume 9(2), pp. 123–128
Cao, C., Xue, Z., Duan, H., 2016. Making
Ferronickel from Laterite Nickel Ore by Coal-Based Self-Reduction and High
Temperature Melting Process. International Journal of Nonferrous Metallurgy, Volume 5(2), pp. 9–15
Carmignano, O.R.D.R., Vieira, S.S., Brandão, P.R.G., Bertoli, A.C., Lago, R.M., 2020.
Serpentinites: Mineral Structure, Properties and Technological Applications. Journal of the Brazilian Chemical
Society, Volume 31, pp. 2–14
Elliott, R., Pickles, C.A., Forster, J., 2016.
Thermodynamics of the Reduction Roasting of Nickeliferous Laterite Ores. Journal
of Minerals and Materials Characterization and Engineering, Volume 4(6), pp. 320–346
Ersoy, B., Dikmen, S., Yildiz, A.,
Gören, R., Elitok, Ö., 2013. Mineralogical and Physicochemical Properties of Talc
from Emirda?, Afyonkarahisar, Turkey. Turkish Journal
of Earth Sciences, Volume 22(4,) pp. 632–644
Farrokhpay, S., Cathelineau, M., Blancher, S.B.,
Laugier, O., Filippov, L., 2019. Characterization of Weda Bay Nickel Laterite Ore from Indonesia. Journal
of Geochemical Exploration, Volume 196, pp. 270–281
Gourley, S.W.D., Or, T., Chen, Z., 2020. Breaking
Free from Cobalt Reliance in Lithium-Ion Batteries. Iscience, Volume 23(9), pp. 1–12
Hosseini Nasab, M., Noaparast, M., Abdollahi, H.,
2020. Dissolution Optimization and Kinetics of Nickel and Cobalt from Iron-rich Laterite Ore, using Sulfuric Acid at Atmospheric Pressure. International
Journal of Chemical Kinetics, Volume 52(4), pp. 283–298
Hosseini, S.A., Raygan, S., Rezaei, A., Jafari,
A., 2017. Leaching of Nickel from a Secondary Source by Sulfuric Acid. Journal of
Environmental Chemical Engineering, Volume 5(4), pp. 3922–3929
Ilyas, A., Kashiwaya, K., Koike, K., 2016. Ni Grade Distribution in Laterite Characterized from Geostatistics, Topography and
the Paleo-groundwater System in Sorowako,
Indonesia. Journal of Geochemical Exploration, Volume 165, pp. 174–188
Ito, A., Otake, T., Maulana, A., Sanematsu, K.,
Sufriadin, Sato, T., 2021. Geochemical Constraints on the Mobilization of Ni and Critical Metals in Laterite Deposits, Sulawesi,
Indonesia: A Mass-balance Approach. Resource Geology, Volume 71(3), pp. 255–282
Kusrini, E., Usman, A., Trisko, N., Harjanto, S.,
Rahman, A., 2019. Leaching Kinetics of Lanthanide in Sulfuric Acid from Low
Grade Bauxite. Materials Today: Proceeding,
Volume 18, pp. 462–467
Kusrini, E., Zulys, A., Rachmana, A., Wulandari,
D.A., Muharam, Y., Usman, A., Rahman, A., 2020. Enrichment and Extraction of Lanthanum from Belitung Silica Sand using Sulfuric Acid Heap Leaching, Precipitation and Complexation with Phytic Acid. Materials Today: Proceeding, Volume 31, pp. 421–425
Kusrini, E., Zulys, A., Yogaswara, A.,
Prihandini, W.W., Wulandari, D.A., Usman, A., 2020. Extraction and Enrichment
of Lanthanide from Indonesian Low Grade Bauxite using Sulfuric Acid Heap
Leaching and Phytic Acid. Engineering journal,
Volume 24(4). pp. 305–314
Levenspiel, O., 1999. Chemical Reaction
Engineering, 3rd ed. In: John Wiley & Sons, Inc., New York
Li, G.H., Rao, M.J., Li, Q.,
Peng, Z.W., Jiang, T., 2010. Extraction of cobalt from laterite ores by citric
acid in presence of ammonium bifluoride. Transactions of Nonferrous Metals
Society of China. Volume 20(8), 1517–1520
Liu, F., Peng, C., Porvali,
A., Wang, Z., Wilson, B.P., Lundström, M., 2019. Synergistic Recovery of
Valuable Metals from Spent Nickel-Metal Hydride Batteries and Lithium-Ion
Batteries. ACS (American Chemical Society) Sustainable Chemistry &
Engineerin, Volume 7(19), pp. 16103–16111
Maciag, L., Zawadzki, D.,
Kozub-Budzyn, G.A., Piestrzynski, A., Kotlinski, R.A., Wróbel, R.J., 2019.
Mineralogy of Cobalt-rich ferromanganese crusts from the Perth Abyssal plain (E
Indian Ocean). Minerals, Volume 9(2), p. 84
Mirwan, A., Susianto,
Altway, A., Handogo, R., 2017. A Modified Shrinking Core Model for Leaching of
Aluminum from Sludge Solid Waste of Drinking Water Treatment. International
Journal of Technology, Volume 8(1), pp. 19–26
Mohammadreza, F., Mohammad,
N., Ziaeddin, S.S., 2014. Nickel Extraction From Low Grade Laterite by
Agitation Leaching at Atmospheric Pressure. International Journal of Mining
Science and Technology, Volume 24(4), pp. 543–548
Ning, Z.Q., Xie, H.W., Song,
Q.S., Yin, H.Y., Zhai, Y.C., 2019. Nickel Leaching From Low-Grade Nickel Matte
Using Aqueous Ferric Chloride Solution. Rare Metals, Volume 38, pp. 1199–1206
Prihutami, P., Prasetya, A.,
Sediawan, W.B., Petrus, H.T.B.M., Anggara, F., 2021. Study on Rare Earth
Elements Leaching from Magnetic Coal Fly Ash by Citric Acid. Journal of
Sustainable Metallurgy. Volume 7(3), pp. 1241–1253
Prihutami, P., Sediawan,
W.B., Petrus, H.T.B.M., Prasetya, A., 2022. A Product Diffusion Model for the
Extraction of Cerium and Yttrium from Magnetic Coal Fly Ash Using Citric Acid
Solution. International Journal of Technology Volume 13(4), pp. 921–930
Purwanto, A., Nisa, S.S.,
Lestari, I.P., Ikhsanudin, M.N., Yudha, C.S., Widiyandari, H., 2021. High
Performance Nickel Based Electrodes in State-of-the-Art Lithium-ion Batteries:
Morphological Perspectives. KONA Powder and Particle Journal, Volume 38,
pp. 130–149
Sagapoa, C.V., Imai, A., Watanabe, K., 2011. Laterization Process of Ultramafic
Rocks in Siruka, Solomon Islands. Journal of Novel Carbon Resource Sciences,
Volume 3, pp. 32–39
Sahu, S., Kavuri, N.C.,
Kundu, M., 2011. Dissolution Kinetics of Nickel Laterite Ore using Different Secondary
Metabolic Acids. Brazilian Journal of Chemical Engineering, Volume 28, pp.
251–258
Senanayake, G., Childs, J.,
Akerstrom, B.D., Pugaev, D., 2011. Reductive Acid Leaching of Laterite and Metal
Oxides - A Review with New Data for Fe(Ni,Co) OOH and a Limonitic Ore. Hydrometallurgy,
Volume 110, pp. 13–32
Setiawan, H., Petrus,
H.T.B.M., Perdana, I., 2019. Reaction Kinetics for Lithium and Cobalt Recovery
from Spent Lithium-Ion Batteries using Acetic Acid. International Journal of
Minerals, Metallurgy, and Materials, Volume 26(1), pp. 98–107
Sufriadin, Widodo, S., Nur,
I., Ilyas, A., Ashari, M.Y., 2020. Extraction of Nickel and Cobalt from
Sulawesi Limonite Ore in Nitric Acid Solution at Atmospheric Pressure. In:
IOP Conference Series: Materials Science and Engineering, Volume 875(1), p.
012053
Sun, Q., Cheng, H., Mei, X.,
Liu, Y., Li, G., Xu, Q., Lu, X., 2020. Efficient Synchronous Extraction of
Nickel, Copper, and Cobalt from Low–Nickel Matte by Sulfation Roasting-Water
Leaching Process. Scientific Reports, Volume 10(1), p. 9916
Sverdrup, H.U., Olafsdottir,
A.H., 2019. Assessing the Long-Term Global Sustainability of the Production and
Supply for Stainless Steel. BioPhysical Economics and Resource Quality, Volume
4, pp. 1–29
Tang, J., Valix, M., 2004.
Leaching of Low- Grade Nickel Ores by Fungi Metabolic Acids. In:
Separations Technology VI: New Perspectives on Very Large-Scale Operations,
ECI (Engineering Conferences Internasional) Digital Archives, pp. 1–16
Trisnawati, I., Prameswara,
G., Mulyono, P., Prasetya, A., Petrus, H.T.B.M., 2020. Sulfuric Acid Leaching
of Heavy Rare Earth Elements (HREEs) from Indonesian Zircon Tailing. International
Journal of Technology, Volume 11(4), pp. 804–816
Tupaz, C.A.J., Watanabe, Y.,
Sanematsu, K., Echigo, T., Arcilla, C., Ferrer, C., 2020. Ni-co Mineralization
in the Intex Laterite Deposit, Mindoro, Philippines. Minerals, Volume 10(7),
p. 579
Van der Ent, A., Baker,
A.J.M., van Balgooy, M.M.J., Tjoa, A., 2013. Ultramafic Nickel Laterites in
Indonesia (Sulawesi, Halmahera): Mining, Nickel Hyperaccumulators and Opportunities
for Phytomining. Journal of Geochemical Exploration, Volume 128, pp. 72–79
Wanta, K.C., Perdana, I.,
Petrus, H.T.B.M., 2016. Evaluation of Shrinking Core Model in Leaching Process
of Pomalaa Nickel Laterite using Citric Acid as Leachant at Atmospheric Conditions.
In: IOP Conference Series: Materials Science and Engineering, Volume
162(1), p. 012018
Wanta, K.C., Astuti, W.,
Perdana, I., Petrus, H.T.B.M., 2020. Kinetic Study in Atmospheric Pressure
Organic Acid Leaching: Shrinking Core Model. Minerals, Volume 10(7) pp.
1–10
Wanta, K.C., Astuti, W.,
Petrus, H.T.B.M., Perdana, I., 2022. Product Diffusion-Controlled Leaching of
Nickel Laterite using Low Concentration Citric Acid Leachant at Atmospheric
Condition. International Journal of Technology, Volume 13(2), pp.
410–421
Xiao, J., Ding, W., Peng,
Y., Chen, T., Zou, K., Wang, Z., 2020. Extraction of Nickel from Garnierite
Laterite Ore using Roasting and Magnetic Separation with Calcium Chloride and Iron
Concentrate. Minerals, Volume 10(4), p. 352
