Development of Efficient and Scalable Production Process of Analytical Grade Sodium Chloride at Laboratory Scale
Published at : 17 May 2024
Volume : IJtech
Vol 15, No 3 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i3.5606
Rismana, E., Arbianto, A.D., Kusumaningrum, S., 2024. Development of Efficient and Scalable Production Process of Analytical Grade Sodium Chloride at Laboratory Scale. International Journal of Technology. Volume 15(3), pp. 743-752
| Eriawan Rismana | Research Center for Pharmaceutical Ingredient and Traditional Medicine, National Research and Innovation Agency, Jl. Raya Bogor Km. 49, Cibinong, West Java 16911, Indonesia |
| Alfan Danny Arbianto | Research Center for Pharmaceutical Ingredient and Traditional Medicine, National Research and Innovation Agency, Jl. Raya Bogor Km. 49, Cibinong, West Java 16911, Indonesia |
| Susi Kusumaningrum | Research Center for Pharmaceutical Ingredient and Traditional Medicine, National Research and Innovation Agency, Jl. Raya Bogor Km. 49, Cibinong, West Java 16911, Indonesia |
Analytical grade Sodium chloride (NaCl) is one type of salt with a purity of > 99.50 % NaCl content, including other impurities. In laboratories, salt is used as a reagent and a standard solution. This research carried out the salt production process through purification, acidification, neutralization, crystallization, and drying methods. Compared to existing technologies, the advantages of this technology are its simplicity and ability to produce accompanying products. In the first stage of the purification process, the precipitation of sulfate (SO42-) and magnesium (Mg2+) ions was examined. In this step, 20 % w/v barium chloride (BaCl2) and 20 % w/v sodium hydroxide (NaOH) solution were consecutively used, and after 12 hours of sitting, the filtering process separated the mixture. The second stage of purification will investigate the precipitation of calcium (Ca2+) ions using 20 % w/v sodium carbonate (Na2CO3) solution, and the mixture was again separated by filtering after 12 hours. All stages of the purification process have shown that the ions concentration decreases by adding BaCl2, NaOH, and Na2CO3 solution. Furthermore, the acidification, neutralization, and crystallization processes can control pH and potassium (K+) content. The results demonstrated that the technology produced 30% analytical grade NaCl with purity > 99.50% and high brine solution as a by-product, which can be further processed for producing pharmaceutical and food-grade salts.
Analytical grade; Development; Production process; Purification; Sodium chloride
Sodium Chloride (NaCl) or salt is an essential commodity. It can be used as a raw material or a mixture in various fields, including chemical, agricultural, animal husbandry, pharmaceutical, health, cosmetic, food, and beverage industries. In the chemical industry, salt is the primary raw material for the production of chlorine gas (Cl2), hydrochloric acid (HCl), sodium hydroxide (NaOH), sodium sulfate (Na2SO4), sodium carbonate (Na2CO3), and sodium bicarbonate (NaHCO3) (Ono, and Erhard, 2012; Sedivy, 2010). Salt is widely used in leather tanning, fish preservation, and oil drilling. Moreover, it is also used in weather modification through seeding and cloud formation to form artificial rain. Types of salt suitable for use include analytical, pharmaceutical, industrial, food, and miscellaneous grade salts. Food-grade salt is known for making salted fish-tanning skin (Ono, and Erhard, 2012; Sedivy, 2009).
Analytical grade NaCl is a high-purity salt with a NaCl content of 99.50 - 100.50% and a low content of other compounds. The most common use of salt is in laboratory chemicals. Its common uses include chemical reagents, standard sodium ion in absorption atomic spectrophotometers, standardization of AgNO3 solutions in argentometry volumetric, microbiological media, material synthesis, cell microbiology, and ion chromatography.
NaCl is
also used for special purposes in the synthesis of material. The effect of
hydrothermal reaction time and NaCl addition on nano rosette TiO2
crystal growth characteristics during hydrothermal reaction has been examined.
With no addition of NaCl, the nano rosette forms at full growth, indicated by
the high intensity of the crystal structure indexed to rutile P42/mnm with
lattice parameters of a = 4.557(6) Å and c = 2.940(5) Å. The cross-sectional
rosette petal grew to 250 nm. On the contrary, the crystal growth during
hydrothermal reaction could be controlled with the addition of NaCl. In this
work, with the addition of 2.5% v/v NaCl, the cross-sectional rosette size was
only about 50 nm, 80% smaller than that of the crystal grown with no NaCl addition
(Sofyan et al., 2019).
Analytical
grade NaCl specifications refer to Sigma Aldrich, Merck, and Fisher Scientific
standards. The quality requirement of the analytical grade NaCl was showed in
Table 1.
Table 1 The quality of requirements for analytical grade NaCl
This process produces
analytical-grade salt. Therefore, the purification stages are necessary to
increase NaCl content and reduce impurities of ions such as sulfate, calcium,
magnesium, and potassium. Therefore, many studies have been conducted to
improve the quality of salt by using several methods. Two of the common
processes for purifying crystalline chemical compounds including salt is using
the crystallization (Lu et al., 2017; Jones, 2002; Giulietti et al., 2001; Mullin, 2001) and precipitation methods (Mersmann,
2004).
These reported methods
include re-crystallization (Rismana and Nizar, 2014; Rositawati, Taslim,
and Soetrisnanto, 2013; Nurhidayati, 2007), hydro extraction (Kharismanto
et al., 2021; Sumada, Dewati, and Suprihatin, 2018; Martina et al., 2016), coagulation (Astuti,
2021; Lukum et al., 2021 Pujiastuti et
al., 2017; Rathnayaka et al., 2014; 2013; Sulistyaningsih, Sugiyo, and Sedyawati, 2010; Ihsan and Djaeni, 2002; Yang, Yun, and Yang, 2007), geomembrane (Hoiriyah,
2019), purification – evaporation (Luo et al., 2022; Schimdt, Meirhofer, and
Schwaiger, 2009; Wae, Tjahjanto, and Mardiana, 2021), simultaneous crystallization
(Penha, Zago, and Seckler, 2020; Zago, Penha, and
Seckle, 2019; Penha et al., 2018), purification using porous material composite (Sulhadi, et al.,
2017) and High-Density Polyethylene (HDPE) membrane (Rusiyanto Soesilowati, and Jumaeri 2013). However, these results are only able to
produce industrial and food-grade salts.
The study of the process of
purification of raw salt is evaluated by washing with a saturated solution of
pure NaCl to remove the impurities. The results showed that washing the salt
with brine can decrease contaminants such as magnesium, calcium, sulfate, and
heavy metal (Ahmmed et al., 2018).
Furthermore, research to improve NaCl quality has been performed by drying the
salt using a hot air-drying system. As a result, the NaCl concentration
increased but could not reduce the impurity content (Amir
et al., 2021). Research for preparing analytical grade salt was
investigated by re-dissolved, coagulation, ion exchange, and re-crystallization
processes from rock salt (Rahman, Islam, and Farrukh, 2010). However, this studied process uses resin to reduce potassium levels,
which is costly when developed on an industrial scale. The preparation of
pharmaceutical-grade salt was also investigated using purification by
precipitation and only one partially crystallized stage from crude salt as raw
material (Rismana, 2016; Rismana and Srijanto, 2013). Nevertheless, the product still has a high
potassium content which does not meet the NaCl analytical grade standard.
The novelty of this study
was to reduce calcium, magnesium, and sulfate impurities using two stages of
precipitation. Three processes of partial re-crystallization were also carried
out to decrease potassium content. In addition, this production process produces
a high-quality salt solution, which can be used as raw material to make
pharmaceutical or food salts. Therefore, developing the process production
technology analytical grade NaCl in Indonesia is still necessary at a
laboratory and bench scale. The benefit of this research is the availability of
the technology process and accessibility that the domestic industry can easily
apply.
2.1. Materials
The materials are divided into raw,
refining, and analysis groups. Raw and refining materials consist of crude
salt, industrial-grade of Na2CO3 (PT. Brataco, Bandung,
Indonesia), industrial-grade of NaOH and 32% HCl (PT Asahi Mas, Cilegon
Indonesia), industrial-grade of BaCl2 (Haihang Industry, Co. Ltd.,
Jinan, China). While, the materials pro
analysis grade for analysis include AgNO3, NaCl K2CrO4,
Na-EDTA, EBT indicator, murexide indicator, 36% w/v HCl, NaOH, blue thymol
bromine indicator, NH4OH, NH4Cl, Na2SO4,
BaCl2, oxalic acid (Merck, Germany).
2.2. Purification and Production Processes of Analytical
Grade NaCl
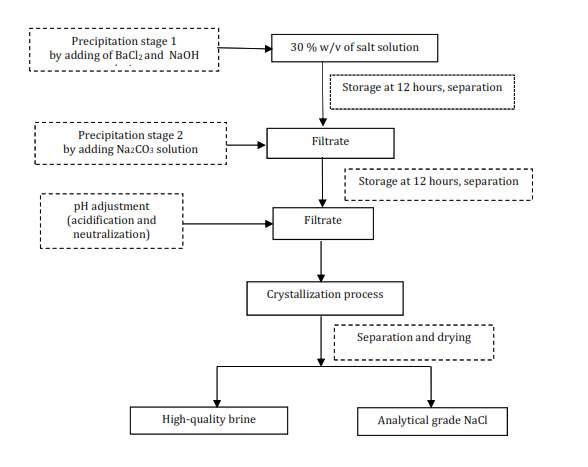
The purification
stages for this process will be used as follows: (1) crude salt was dissolved
with water to obtain a brine solution with NaCl content of about 27-30 % w/v, (2) the
brine solution was then filtered to remove insoluble impurities, (3) analysis
of NaCl, SO42-, Mg2+, Ca2+,
water-insoluble content, and water content is carried out on the sample of the
initial brine solution, the brine solution after purification, after filtering
stages, and the analytical grade NaCl product, (4) purification and filtering
process, (5) acidification and neutralization, (6) crystallization, and (7)
separation and drying process. Figure 1 shows a complete set of production
processes.
The purification
process was performed using the precipitation of SO42-
with BaCl2, Mg2+ with NaOH, and Ca2+ with Na2CO3
using the two-stage method. The first process was examined by coagulating SO42-
and Mg2+ ions using 20 % w/v BaCl2 and NaOH solution
successively, and after 12 hours left, the mixture was separated by the
filtering process. The second process was studied by precipitation of Ca2+
ion using 20 % w/v Na2CO3 solution, and after 12 hours,
the mixture was separated by the filtering process again. The detailed
procedure was as follows; the brine solution was reacted with a 20% w/v BaCl2
solution until the SO42- the content was < 0.001 % (10
ppm). Furthermore, a brine solution containing BaSO4(s) was added
20% w/v NaOH to Mg2+ content of < 0.001 % (10 ppm). The next step
was to separate BaSO4(s) and Mg (OH)2(s) by filtering.
Figure 1 Schematic diagram of process production analytical grade NaCl by
two stages of precipitation
2.3. Product Characterizations and Analysis
Product and intermediate NaCl were investigated according to the standard CoA of NaCl analytical grade. The NaCl content was calculated based on the chloride ion content determined by argentometry volumetric. While the calcium, magnesium, potassium, and heavy metal ions content were analyzed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) spectrophotometer. A pH solution was determined using a pH meter, and the Loss on Drying (LOD) apparatus measured water content. While sulfate content was determined using a turbidimetry spectrophotometer.
3.1. Analysis of Salt Raw Materials and 27-30 % w/v Initial Brine Solution
The quality of salt raw material needs to be analyzed to determine whether it meets the specification requirements. Table 2 shows the results of the analysis of NaCl content and the main impurities of the salt raw material. The analysis results in Table 2 show that raw salt materials have 87.30 % NaCl content. This salt indicates that the raw material still has low NaCl content. However, the calcium, magnesium, and sulfate impurities content is standard and meets consumption salt specifications. The content of water-insoluble impurities is relatively low; it will facilitate the separation of contaminants during the filtering process to get a clear salt solution.
The higher NaCl and lower impurities compound content in salt raw material will be advantageous for producing industrial-grade salt (Chlor Alkali Plant (CAP), pharmaceutical, and analytical grade) due to fewer materials needed for refining and higher-yield products. The salt is dissolved in water to obtain a brine solution with a NaCl concentration of about 27-30% w/v. Adding water to the 27-30% salt solution also causes lower levels of calcium, magnesium, and sulfate impurities. Table 3 shows the analysis results of several main parameters of the initial brine solution.
3.2. Production of Brine Solution after Purification and NaCl Analytical Grade
Analyzing impurity ions in the brine solution is essential to determine the number of chemicals needed during refining. Table 4 and Figure 2 show the results of the NaCl content analysis and the brine solution's main impurities after purification. In this research, the purification process was carried out in two stages: the first stage of precipitation using 20 % w/v BaCl2 and NaOH to reduce the sulfate (SO42-) and magnesium (Mg2+) ions content. In contrast, to reduce calcium (Ca2+) ion content, we studied the second coagulation stage by adding 20 % w/v Na2CO3 to reduce the calcium (Ca2+) ion content.
In the first stage, the amount of SO42- and Mg2+ ions were decreased after precipitation with 20 % w/v BaCl2 and NaOH at concentrations 0.0005-0.0006 % (5-6 ppm) and 0.0008-0.0012 % (8-12 ppm), respectively. The Ca2+ content did not significantly reduce because calcium did not react with NaOH and BaCl2. After storage for 12 hours and filtration, the SO42- and Mg2+ ions content remained relatively unchanged.
In the second precipitation stage, a reaction with 20 % w/v Na2CO3 reduced the concentration of Ca2+ ion to 0.0015 % (15 ppm). In contrast, SO42- and Mg2+ ion content after 12 hours of storage and filtering showed a similar concentration. The NaCl concentration decreased from 26.30 % to 25.55 % after the first and second stage processes due to the dilution process of adding reagents. As presented below in Table 4, replicating three batches of the purification process showed good repeatability.
In the following process, HCl and NaOH solutions were added to control the pH brine solution in the acidification and neutralization stage. First, HCl solution is added, so the brine solution's pH becomes 2-3. Furthermore, the pH was raised again to pH 5-6 by adding a solution of NaOH. Adjusting the pH brine solution is essential to remove carbonate content so the product can meet the standard acidity specifications. Therefore, decreasing the pH to 2-3 in the salt solution was done to remove residual carbonate ions that should not be present in the NaCl pro analysis. While increasing the pH after that process to pH 5-6 is to return the pH of the solution to be neutral so that the final NaCl product can meet the pH requirements of 6-7 when testing the pH of the solution. challenging.
3.3. Production of Analytical Grade NaCl Product
The process was validated by a triplicate. Table 5 presents the yield and analysis of product results. The average yield of the process production was about 30.33 %. Meanwhile, the quality of the product also met the requirements of the main parameters of the salt analytical grade specifications. In addition to calcium, magnesium, sulfate, and potassium impurities, barium must also be tested as impurities because it is a heavy metal and one of the reagents added as BaCl2 to meet the requirements according to the standard.
One advantage of this process is that the outcome by-products of high-quality brine solution can be used as a raw material for producing pharmaceutical and food-grade salts. Table 6 shows the analysis of pharmaceutical-grade salt after crystallization and drying from high-quality residual brine. The pharmaceutical-grade salt product offers good quality with NaCl, Ca2+, SO42- and Mg2+ contents and meets the requirements set by pharmacopeia.
This research carried
out the salt production process through purification, acidification,
neutralization, crystallization, separation, and drying methods. This developed
technology process of analytical grade salt production at the laboratory scale
has produced salt according to the specifications required, with an average
product yield of 30.33 % and a purity of NaCl of 100.15 %. Its simplicity and
ability to produce accompanying products. Compared to existing technologies,
the advantages of this production technology are its simplicity and ability to
make multiple accompanying products. Moreover, by-products of high-quality
brine in this technology can be used as raw material to produce pharmaceutical
and food-grade salt. Therefore, this invention has excellent potential to
accommodate domestic demands, substitute imported products, and increase the economic
value of crude salt as raw material.
The authors would
like to thank the PT Karya Darya Syafarmasi for the research collaboration and
application of the innovation at an industrial scale.
Ahmmed, S.M., AbdElaziz, H., Mergani, S., Alabed,
A., 2018. Effect of Purification on Sodium Chloride produced from Seawater
Based on Solar Evaporation. Red Sea University Journal of Basic and Applied
Science, Volume 2, pp. 37–44
Amir, N., Efendy, M., Yoo, Y.J., Gozan, M., 2021.
Improved Salt Quality and Reduced Energy Consumption via Hot Air Drying. International
Journal of Technology. Volume 12(3), pp. 592–601
Astuti, D.H., Purba, T., Su’udi, N., Sani,
Nugraha, R.E., 2021. The Role of Na2CO3 as Precipitating Agent in Salt
Purification Process. In: 2nd
International Conference Eco-Innovation in Science, Engineering, and
Technology. NST Proceedings, pp. 25–29
Giulietti, M., Seckler M.M., Derenzo, S., Ré,
M.I., Cekinski, E., 2001. Industrial Crystallization and Precipitation from
Solutions: State of the Technique. Brazilian
Journal of Chemical Engineering, Volume
18, pp. 423–440
Hoiriyah, Y.U., 2019. Improving the Quality of
Salt Production Using Technology Geomembrane. Jurnal Studi Manajemen dan Bisnis, Volume 6(2), pp. 71–6
Ihsan, D., Djaeni, M., 2002. Improving Public
Salt Quality by Chemical Treatment. Journal
of Coastal Development, Volume 5(3), pp. 111–116
Jones, A.G., 2002. Crystallization Process
Systems. Amsterdam: Elsevier
Kharismanto, B., Triandini, R., Triana, N.W.,
Suprihatin, S., 2021. Purification of Crude Solar Salt into Industrial Salt
Using a Hydro Extractor. Chempro, Volume
2(02), pp. 24–30
Lu, H., Wang, J., Wang, T., Wang, N., Bao, Y.,
Hao, H., 2017. Crystallization Techniques in Wastewater Treatment: An Overview
of Applications. Chemosphere, Volume 173,
pp. 474–484
Lukum, A ., Mohamad, E, Tangahu, A.D.K., Ohi,
S.Y., 2021. Production and Optimization of Sea Salt Quality on the Coast of
Tomini Bay. Journal of Physics:
Conference Series, Volume 1968(1),
p. 012013
Luo, X., Li, X., Wei, C., Deng, Z., Liu, Y., Li,
M., Zheng, S., Huang, X., 2022. Recovery of NaCl and Na2SO4
from High Salinity Brine by Purification and Evaporation. Desalination, Volume 530, p. 115631
Martina, A., Witono, J.R., Pamungkas, G.K., Willy, 2016. Effect of Raw Material Quality
and Feed Ratio to Solvent on Salt Refining Process Using Batch Hydroextraction.
Jurnal Teknik Kimia (Journal of Chemical
Engineering), Volume 5 (1), pp. 1–6
Mersmann, M.K,, 2004. Chemical Engineering
Aspects of Precipitation from Solution. Chemical
Engineering & Technology, Volume 11, p. 264–276
Mullin, J.W., 2001. Crystallization. 4th
Edition. Oxford Boston: Butterworth-Heinemann
Nurhidayati, L., 2007. Improving the Quality of
Sodium Chloride from Table Salt by Partial Re-crystallization. In:
National Seminar on Chemistry, University of Indonesia
Ono, K., Erhard, A,, 2012. Ullmann’s Encyclopedia
of Industrial Chemistry. Wiley – VCH
Penha, F.M., Zago, G.P., Seckler, M.M., 2020.
Strategies to Control Product Characteristics in Simultaneous Crystallization
of NaCl and KCl from Aqueous Solution: Seeding with NaCl and KCl. Crystal Growth & Design, Volume
19(2), pp. 1257–1267
Penha, M., Zago, G.P., Nariyoshi, Y.N., Bernardo,
A., Seckler, M.M., 2018. Simultaneous Crystallization of NaCl and KCl from
Aqueous Solution: Elementary Phenomena and Product Characterization. Crystal Growth & Design, Volume 18(3), pp. 1645–1656
Pujiastuti, C., Ngatilah, Y., Sumada, K., Muljani, S., 2017. The Effectiveness of
Sodium Hydroxide (NaOH) and Sodium Carbonate (Na2CO3) on
the Impurities Removal of Saturated Salt Solution. In: The 2nd
International Joint Conference on Science and Technology (IJCST), Volume
953(1), p. 012215
Rahman, A., Islam, M.A., Farrukh, M.A., 2010.
Preparation of Analytical Grade Sodium Chloride from Khewra Rock Salt. World Applied Sciences Journal, Volume
9(11), pp. 1223–1227
Rathnayaka, D. D.T., Vidanage, P. W., Wasalathilake, K.C., Perera, S. A.S.,2014 .
Development of a Process to Manufacture High Quality Refined Salt from Crude
Solar Salt. International Journal of
Advances in Chemical Engg., & Biological Sciences (IJACEBS), Volume 1, pp.
137–142
Rathnayaka, D.D.T, Vidanage, P.W., Wasalathilake,
K.C., Wickramasingha, H.W., Wijayarathne, U.P.L., Perera, S.A.S., 2013.
Development of a Process to Manufacture High-Quality Refined Salt from Crude
Solar Salt. International Journal of
Chemical and Molecular Engineering, Volume 7 (12), pp. 1009–1014
Rismana, E., 2016. Determination of Bacterial,
Yeast and Fungi Contaminants at Production Unit of Salt Pharmaceutical at Pilot
Scale 5 Kg/Batch Capacity. Media
Penelitian dan Pengembangan Kesehatan (Health Research and Development Media),
Volume 26(1), pp. 29–36
Rismana, E., Nizar., 2014. The Assessment of
Production Process of Food Grade Salt Using Some Raw Materials Salt. Chemical Progress, Volume 7(1), pp.
25–28
Rismana, E., Srijanto, B., 2013. Development
of Production Technology of Pharmaceutical and Food Grade Salt at BPPT (Agency
for the Assessment and Application of Technology). Research Center for
Science and Technology, University of Indonesia
Rositawati, A.L., Taslim, C.M., Soetrisnanto, D.,
2013. Recrystallization of Crude Solar Salt from Demak Area to Achieve SNI for
Industrial Salt. Jurnal Teknologi Kimia
dan Industri, Volume 2(4), pp. 217–225
Rusiyanto, R., Soesilowati, E., Jumaeri, J.,
2013. Strengthening the National Salt Industry Through Improvement of
Cultivation Technology and Product Diversification. Sainteknol: Jurnal Sains
dan Teknologi, Volume 11 (2), pp. 129–143
Schimdt, J.-H., Meirhofer, W., Schwaiger, H.,
2009. Process Optimization of Brine Purification and Evaporation for Combined
Crystallization of NaCl and Na2SO4 by Means of Mechanical
Vapour Recompression. In: 9th International Symposium Salt,
Volume 2009
Sedivy, V.-M., 2009. Environmental Balance of
Salt Production Speaks in Favour of Solar Saltworks. Global Network of Environmental Science and Technology Journal,
Volume 11(1), pp. 41–48
Sedivy, V.-M., 2010. Processing of Salt for
Chemical and Human Consumption. In: 9th International Symposium on Salt,
Volume 2, pp. 1385–1402
Sofyan, N.,
Ridhova, A., Yuwono, A.H., Sianturi, M.C., 2019. Effect of NaCl Addition on Nano Rosette
TiO2 Crystal Growth During Hydrothermal Deposition. International Journal of Technology, Volume 10(6), pp. 1235–1242
Sulhadi, Susanto, Priyanto, A., Fuadah, A.,
Prasetya, M.A., 2017. Performance of Porous Composite from Waste Glass on Salt
Purification. Process Procedia
Engineering, Volume 170, pp. 41–46
Sulistyaningsih, T., Sugiyo, W., Sedyawati,
S.M.R., 2010. Purifying Food Salt by Crystallization Methods from Saturated
Salt Solution by Chemical Treatment using Na2C2O4-NaHCO3
dan Na2C2O4-Na2CO3. Sainteknol:
Jurnal Sains dan Teknologi, Volume 8(1), pp. 26–33
Sumada, K., Dewati, R., Suprihatin, 2018.
Improvement of Seawater Salt Quality by Hydroextraction and Re-crystallization
Methods. Journal of Physics: Conference
Series, Institute of Physics (IOP) Publishing, Volume 953(1), p. 012214
Wae, E., Tjahjanto, R.T., Mardiana, D., 2021. Study of Sodium Chloride Production
Using Gradual Evaporation of Seawater from Nggolonio Sea, East Nusa Tenggara. The Journal of Pure and Applied Chemistry
Research, Volume 10(2), pp.
123–131
Yang, W., Yun, Y., Yang, L., 2007. Experimental
Study on Brine Purification with “Lime-flue gas” Process, China Well Rock Salt, Volume 38(5), pp. 29–31
Zago, G.P., Penha, F.M., Seckle, M.M., 2019.
Product Characteristics in Simultaneous Crystallization of NaCl and CaSO4
from Aqueous Solution under Different Evaporation Rates. Desalination, Volume 457, pp. 85–89
