Innovation Options and Profitability of Pharmaceutical Brand Manufacturers
Published at : 07 Oct 2022
Volume : IJtech
Vol 13, No 4 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i4.5561
Riedel, N.H., Špacek, M., 2022. Innovation Options and Profitability of Pharmaceutical Brand Manufacturers. International Journal of Technology. Volume 13(4), pp. 890-899
| Norman Hendrik Riedel | University of Economics in Prague, Winston Churchill Sq. 4, 130 67, Praha 3, Prague, Czech Republic |
| Miroslav Špacek | University of Economics in Prague, Winston Churchill Sq. 4, 130 67, Praha 3, Prague, Czech Republic |
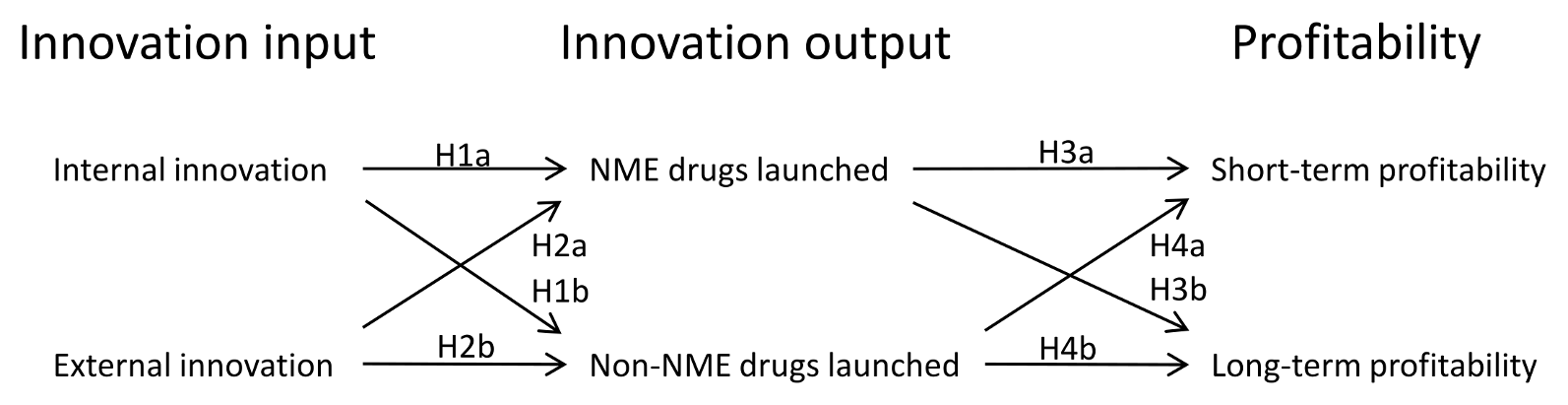
Much research exists
covering clinical development success rates, development costs of new drugs,
and market launch impact on stock market valuation of companies, but little
systematic work has been done to establish the impact of research input on new
product launches and, in turn, their impact on profitability of drug
manufacturers. This article investigates these relations using data from the
world’s largest pharmaceutical brand manufacturers and their product launches
in the US over a period of more than 25 years. The objective is to determine
the impact of innovation intensity on innovation output intensity and of
innovation output intensity on profitability. It is shown that there is a
complete lack of evidence that launches of New Molecular Entities (NMEs)
necessarily lead to higher profitability, suggesting that many launches of NMEs
are not particularly successful from an economic point of view. Furthermore, it
was found that intangible knowledge assets acquired by company mergers and
acquisitions do often not live up to their valuation. This leads to the
conclusion that such intangible assets seem to be overpriced on average. The
more and more frequently used strategy of launching new drugs without NMEs like
combination drugs or extension of indications increased short-term
profitability making this a valid approach to avoid setbacks when patent
protection of blockbuster drugs expires.
Drugs; Pharmaceutical industry; Product innovation; Profitability; Research and development
The
importance of pharmaceutical product innovation for human life has been
demonstrated many times, e.g., by the factual elimination of many
life-threatening diseases like the plague, tuberculosis and smallpox. Vaccine
development during the recent pandemic once more showed the importance and the
capabilities of pharmaceutical development as well as the enormous development
costs and high risks of failure. There have been many investigations on how to
create innovation (Berawi, 2021), improve certain drug formulations (Timotius et al., 2022), increase sustainability (Zaytsev et al., 2021), as well as improve drug supply chains (Goodarzian et al.,
2021). However, this vast amount of literature does
not address the issue that therapeutic and economic success of a new drug are separate
things, despite sometimes staggering prices for new drug therapies. Drug
development has always been known to be notoriously difficult and time-consuming
(Scherer, 2010). Recently there is a growing
concern about the profitability of market introduction of new drugs originating
from longer development times and shorter market exclusivity for companies
developing such drugs (Berndt et al., 2015;
Lietzan, 2018). On the other hand, consumers and politics complain
about rising drug expenditures and high drug prices by far exceeding
development costs (Costantini & Walensky, 2020;
Fleming, 2019). Considering both
perspectives and their potential implications corporate management needs to
carefully access options and the related risks and potential business
performance to adapt development direction and innovation strategy accordingly (Pisano, 2015). This work aims at providing guidance
to this assessment by quantifying the impact of different choices for
innovation on corporate profitability.
The
outcome of this investigation suggests a lack of sufficiency of product
innovation in terms of NME drug launches for increased profitability. While
successful innovation in terms of market launches of new products might be
considered a necessary condition for increased profitability, other conditions
must be fulfilled as well for economic success. Furthermore, the results show
the limited prognostic power of companies or their employees and the high risk
of technological and economic failure of drugs containing new molecular
entities. From a management point of view, the main implication is that
intangible assets acquired by company mergers and acquisitions, assets swaps,
or licensing deals might be considerably overpriced, suggesting a more careful
approach to intangible asset valuation. However, external acquisition of
proprietary technology can still be beneficial or even necessary for product
innovation. Launching drugs without NMEs can be considered as one measure to
increase short-term profitability of brand manufacturers, making this a
potential approach to avoid setbacks when patent protection of blockbuster
drugs expires.
The publication was supported by an internal grant for the research project VŠE IGS 58/2020.
| Filename | Description |
|---|---|
| R1-CE-5561-20220411033907.png | Figure 2 |
Artz, K.W., Norman, P.M., Hatfield, D.E., Cardinal, L.B., 2010. A
Longitudinal Study of The Impact Of R&D, Patents, and Product Innovation on
Firm Performance. Journal of Product Innovation Management, Volume 27(5),
pp. 725–740
Ball, G. P., Shah, R., Wowak, K. D., 2018. Product Competition,
Managerial Discretion, and Manufacturing Recalls in the U.S. Pharmaceutical
Industry. Journal of Operations Management, Volume 58-59, pp. 59–72
Berawi, M.A., 2021. Philosophy of Technology Design: Creating
Innovation and Added Value. International Journal of Technology, Volume 12(3),
pp. 291–319
Berndt, E.R., Nass, D., Kleinrock, M., Aitken, M., 2015. Decline in
Economic Returns from New Drugs Raises Questions About Sustaining Innovations. Health
Affairs, Volume 34(2), pp. 245–252
Bockova, N., Zizlavsky, O., 2016. Innovation and Financial
Performance of a Company: A Study from Czech Manufacturing Industry. Transformations
in Business & Economics, Volume 15(3), pp. 156–175
Costantini, S., Walensky, R.P., 2020. The Costs of Drugs in
Infectious Diseases: Branded, Generics, and Why We Should Care. The Journal
of Infectious Diseases, Volume 221(5), pp. 690–696
DiMasi, J.A., Grabowski, H.G., Hansen, R.W., 2016. Innovation in The
Pharmaceutical Industry: New Estimates of R&D Costs. Journal of Health
Economics, Volume 47, pp. 20–33
Drugs FDA., 2020. Food and Drug Administration. Available online at
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm, Accessed on May 05,
2021
Dubois, P., Mouzon, O. de, Scott-Morton, F., Seabright, P., 2015.
Market Size and Pharmaceutical Innovation. The RAND Journal of Economics,
Volume 46(4), pp. 844–871
Ernst, H., 2001. Patent Applications and Subsequent Changes of
Performance: Evidence from Time-Series Cross-Section Analyses on The Firm Level.
Research Policy, Volume 30(1), pp. 143–157
Ernst, H., Conley, J., Omland, N., 2016. How To Create Commercial
Value from Patents: The Role of Patent Management. R&D Management, Volume
46, pp. 677–690
Fazl?o?lu, B., Dalg?ç, B., Yereli, A.B., 2019. The Effect of
Innovation on Productivity: Evidence from Turkish Manufacturing Firms. Industry
and Innovation, Volume 26(4), pp. 439–460
Feyzrakhmanova, M., Gurdgiev, C., 2016. Patents and R&D Expenditure
Effects on Equity Returns in Pharmaceutical Industry. Applied Economics
Letters, Volume 23(4), pp. 278–283
Fleming, S., 2019. Drug Prices and Innovation. Forbes. Available
online at https://www.forbes.com/sites/stanfleming/2019/06/20/the-relationship-between-drug-prices-and-innovation/?sh=45a9bbd84b11,
Accessed on January 20, 2022
Garzón-Vico, A., Rosier, J., Gibbons, P., McNamara, P., 2020. The
Impact of Failure and Success Experience on Drug Development. Journal of
Product Innovation Management, Volume 37(1), pp. 74–96
Goodarzian, F., Wamba, S.F., Mathiyazhagan, K., Taghipour, A., 2021.
A New Bi-Objective Green Medicine Supply Chain Network Design under Fuzzy
Environment: Hybrid Metaheuristic Algorithms. Computers & Industrial
Engineering, Volume 160, p. 107535
Leahy, A.S., 2011. The Determinants of Profitability in the
Pharmaceutical Industry. American Journal of Health Sciences (AJHS), Volume
3(1), pp. 37–42
Lietzan, E., 2018. The Drug Innovation Paradox. Missouri Law
Review, Volume 83(1), p. 39
Light, D.W., Warburton, R., 2011. Demythologizing the High Costs of
Pharmaceutical Research. Bio Societies, Volume 6(1), pp. 34–50
Munos, B., 2009. Lessons from 60 Years of Pharmaceutical Innovation.
Nature Reviews Drug Discovery, Volume 8(12), pp. 959–968
OECD, 2019. Health at a Glance 2019 (p. 243). Available online https://doi.org/10.1787/4dd50c09-en,
Accessed on 07/26/2021
Pisano, G.P., 2015. You Need an Innovation Strategy. Harvard
Business Review, Volume 93(6), pp. 44–54
Riedel, N.H., 2021. Impact of Strategic Changes on R&D
Efficiency in Drug Development. In: International
Society for Professional Innovation Management (ISPIM)
Robey, S., David, F.S., 2017. Drug Launch Curves in The Modern Era.
Nature Reviews Drug Discovery, Volume 16(1), pp. 13–14
Saint-Hilary, G., Robert, V., Gasparini, M., 2018. Decision-making
in Drug Development Using a Composite Definition of Success. Pharmaceutical
Statistics, Volume 17(5), pp. 555–569
Scherer, F.M., 2010. Pharmaceutical
Innovation. In B. H. Hall & N. Rosenberg (Eds.), Handbook of The Economics of Innovation,
Volume 1, pp. 539–574, North-Holland
Schuhmacher, A., Gassmann, O., Hinder, M., 2016. Changing R&D Models
in Research-Based Pharmaceutical Companies. Journal of Translational
Medicine, Volume 14(1), pp. 1-11
Schuhmacher, A., Wilisch, L., Kuss, M., Kandelbauer, A., Hinder,
M., Gassmann, O., 2021. R&D Efficiency of Leading Pharmaceutical Companies
– A 20-year Analysis. Drug Discovery Today, Volume 26(8), pp. 1784–1789
Shaikh, M., Del Giudice, P., Kourouklis, D., 2021. Revisiting the
Relationship Between Price Regulation and Pharmaceutical R&D Investment. Applied
Health Economics & Health Policy, Volume 19(2), pp. 217–229
Sood, N., Mulligan, K., Zhong, K., 2021. Do Companies in The
Pharmaceutical Supply Chain Earn Excess Returns? International Journal of
Health Economics Management, Volume 21(1), pp. 99–114
Taalbi, J., 2017. What Drives Innovation? Evidence from Economic
History. Research Policy, Volume 46(8), pp. 1437–1453
Tay-Teo, K., Ilbawi, A., Hill, S.R., 2019. Comparison of Sales
Income and Research and Development Costs for FDA-Approved Cancer Drugs Sold by
Originator Drug Companies. JAMA Network Open, Volume 2(1), p. 186875
Teramae, F., Makino, T., Sengoku, S., Lim, Y., Natori, T., Kodama,
K., 2020. Research on Pharmaceutical Product Life Cycle Patterns for
Sustainable Growth. Sustainability, Volume 12(21), p. 8938
Timotius, D., Kusumastuti, Y., Rochmadi, R., 2022. Characterization
and Equilibrium Study of Drug Release of pH-Responsive Chitosan-graft-Maleic
Film. International Journal of Technology, Volume 13(2), pp. 291–319
Visnjic, I., Wiengarten, F., Neely, A., 2016. Only the Brave:
Product Innovation, Service Business Model Innovation, and Their Impact on
Performance. Journal of Product Innovation Management, Volume 33(1), pp.
36–52
World Bank Open Data, 2020. World Bank. Available online at https://data.worldbank.org/, Accessed on January
10, 2021
Zaytsev, A., Dmitriev, N., Rodionov, D., Magradze, T., 2021. Assessment of the Innovative Potential of Alternative Energy in the Context of the Transition to the Circular Economy. International Journal of Technology, Volume 12(7), pp. 1328–1338
Zeukeng, M.J., Seoane-Vazquez, E., Bonnabry, P., 2018. A comparison of new drugs approved by the FDA, the EMA, and Swissmedic: An Assessment of the international Harmonization of Drugs. European Journal of Clinical Pharmacology, Volume 74(6), pp. 811–818
