Development of Sweat and Saliva Glucose Sensors as Alternative for Non-Invasive Blood Glucose Monitoring
Corresponding email: prasetyanto@atmajaya.ac.id
Published at : 17 May 2024
Volume : IJtech
Vol 15, No 3 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i3.5535
Lukito, A.A., Tyrayoh, M., Prasetyanto, E.A., Rukmini, E., 2024. Development of Sweat and Saliva Glucose Sensor as Alternative for Non-Invasive Blood Glucose Monitoring. International Journal of Technology. Volume 15(3), pp. 720-731
| Agrevina Ane Lukito | School of Medicine and Health Science, Atma Jaya Catholic University of Indonesia, 2 Pluit Raya str., North Jakarta 14440, Indonesia |
| Michelle Tyrayoh | School of Medicine and Health Science, Atma Jaya Catholic University of Indonesia, 2 Pluit Raya str., North Jakarta 14440, Indonesia |
| Eko Adi Prasetyanto | Department of Pharmacy, School of Medicine and Health Science, Atma Jaya Catholic University of Indonesia, 2 Pluit Raya str., North Jakarta 14440, Indonesia |
| Elisabeth Rukmini | Department of Civil Engineering and Center for Urban Studies, Pembangunan Jaya University, Cendrawasih Raya str., South Tangerang 15413, Indonesia |
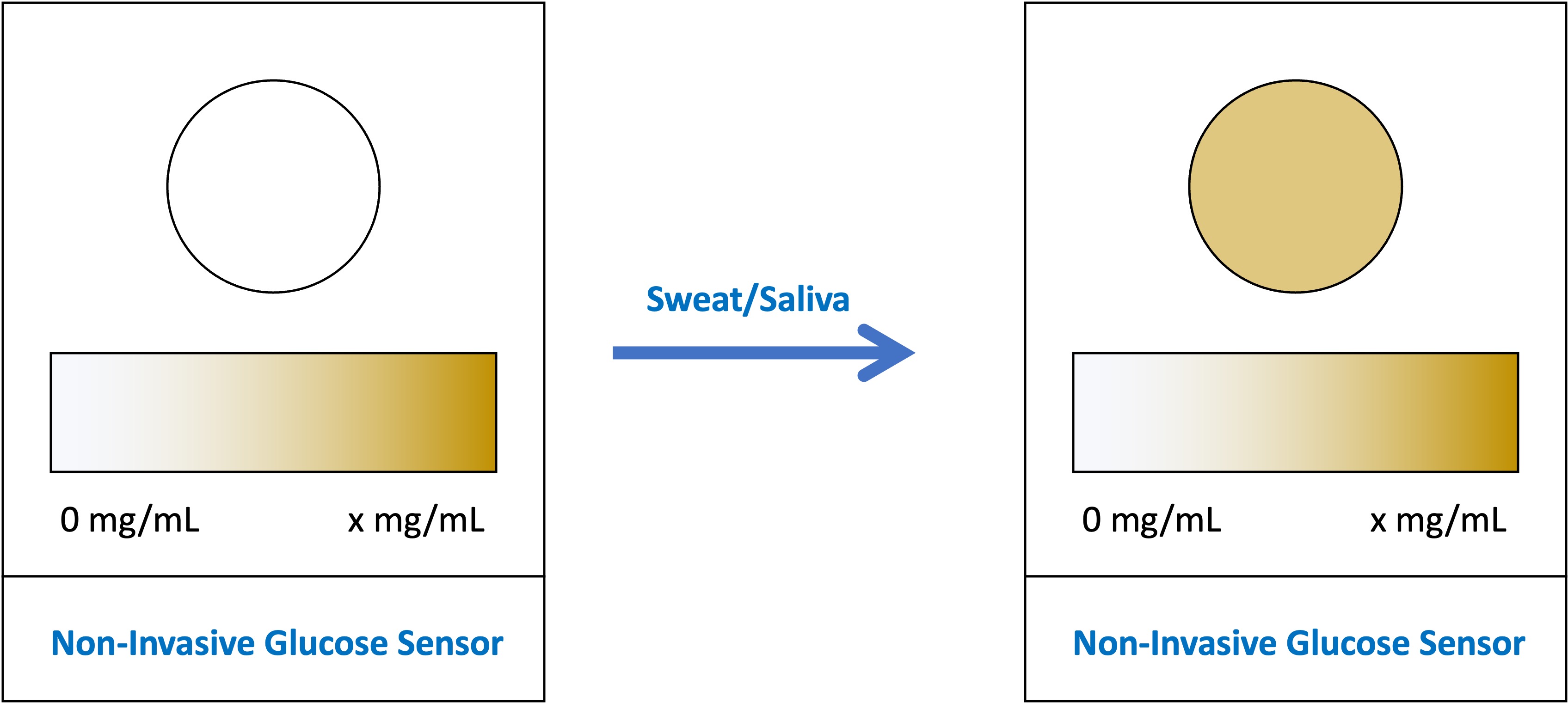
. This study aimed to
develop a paper-based glucose sensor using artificial sweat and saliva samples,
a non-invasive, practical, and affordable concept for measuring glucose levels.
The chosen method was the colorimetric method using the Glucose Oxidase (GOx)
enzyme, Horseradish Peroxidase (HRP) enzyme, and 3,3?-diaminobenzidine (DAB)
chromogen. The wax stamping method was utilized on a piece of filter paper to
test the glucose sensor. The test was carried out in several stages, including
testing various concentrations of reagents and wax stamping sizes, fabricating
the glucose sensors, and testing multiple concentrations of glucose, artificial
sweat, and artificial saliva. The RGB method was used to test the resulting
color. The data obtained were then validated using UV-Vis Spectrophotometry. The
GOx concentration on the glucose sensor affected the color change velocity. The
HRP, DAB, and wax stamping size concentration simultaneously indicated a strong
correlation with changes in the color intensity that appeared on the glucose
sensor. The glucose sensor in this study could measure the glucose
concentration of the artificial sweat and saliva samples, respectively, from
0-3000 and 0.9987. This study proved a
strong correlation between the reagents' concentration and the color intensity
on the developed glucose sensor.
Colorimetry; Glucose; Non-invasive; Saliva; Sweat
Diabetes Mellitus (DM) is
a global epidemic disease that affects blood glucose levels. According to the
2019 International Diabetes Federation (IDF) Diabetes Atlas, around 463 million
adults worldwide, whose ages range from 20-79 years old, suffer from DM. In
Indonesia, there are more than 10 million diabetes cases in adults, with a
prevalence of 6.2% (IDF, 2019).
Self-monitoring blood glucose is important for diabetics, which consists of
controlling sugar levels with the necessary diet and physical activity (Gordon,
2019; Meetoo et al., 2018; Dewi et al., 2017).
This study aimed to design a
non-invasive concept to measure the glucose levels in the body using
paper-based colorimetric methods. The developed device design is a practical,
harmless, and affordable device to simplify the process of routine glucose checks
by the entire community. In order to develop the design concept of the device,
it is essential to test both the reagents concentration, which is required to
measure the glucose levels optimally and the size of wax stamping, which is
required to accommodate the optimal reagents and samples. After obtaining the
most optimal concentration and size of wax stamping, the research was continued
using a paper-based glucose sensor. The glucose sensor was then used to test
samples of several concentrations of pure glucose, glucose in sweat, and
glucose in saliva using the RGB method. The study ended by validating the RGB
findings using UV-Vis Spectrophotometry in order to obtain more accurate and reliable data.
2.1. Materials and equipment
The
materials used to measure the glucose level included Glucose Oxidase (GOx),
Horseradish Peroxidase (HRP) as the enzyme, a chromogen namely
3,3'-diaminobenzidine (DAB), Chitosan powder as the solute, PBS buffer,
glucose, lactic acid, acetic acid, distilled water as the other solution, the
PBST solution which composed of 50 mM PBS, 0.9% NaCl, 0.1% Triton X-100, pH
7.4, and other compounds that consisted of NaCl, NH4Cl, NaOH, KCl,
CaCl2, NaHCO3, and Whatman filter paper no.4 whose
diameter of 110 mm.
The equipment used to measure the glucose level required some devices
consisting of a smartphone with Color Grab™ application (Lookmatix,
2020), several wax stamps, a black
box, some hot plates, an oven, an analytical scale with 0.001 mg precision, and
the UV-Vis Spectrophotometer.
2.2. Research procedures
2.2.1. Testing
with Various Reagent Concentrations
This research used several
concentrations, including GOx, HRP, and DAB, to start the test. In each trial
of a reagent concentration, the other reagents were used as the constant
(identical concentrations). First, a 5 L solution of GOx and a 5
L solution of HRP were
dropped into the sample reservoir on the filter paper until it dried (20
minutes) before the DAB (5
L) was
added. After the DAB dried, the same concentration of glucose solution (20
L) was dripped on each reservoir sample. The filter paper was then
incubated at room temperature for 20 minutes, followed by taking photographs of
the test results with the Color Grab™ application to obtain the RGB values. The
test was finalized by recording the results of each reaction to get the most
optimal concentrations of GOx, HRP, and DAB.
2.2.2. Testing of Wax
Stamping Sizes
After the most optimal GOx,
HRP, and DAB concentrations were obtained, the next step was to test various
diameters of the sample reservoir in the wax stamping. This step was conducted
to get the same volume of samples and reagents in each experiment. The first
step was to melt the paraffin at 130oC,
followed by heating the wax stamp on the melted hot paraffin to be attached to
the wax stamp. Later on, the pattern was formed by pressing the wax stamp on
the filter paper for 5 seconds. Following this, the filter paper was preheated
in the oven for 10 seconds at 100oC (if the
paraffin did not penetrate the back side of the filter paper). After the filter
paper was ready, the reagent and glucose were dropped into the sample reservoir
and incubated in the filter paper for 20 minutes at room temperature. Finally,
the pictures of the resulting color were taken using the Color Grab™
application, and the results were recorded.
2.2.3. Fabrication of
the Glucose Sensor
The fabricating process of
the paper-based glucose sensors began by preparing the filter paper in a round
shape, then printing a hydrophobic barrier with paraffin and anticipating the
complete absorption. Subsequently, the Chitosan was prepared in acetate
solution (5 L) to be dropped on the
sample reservoir until it dried at room temperature. Afterward, the other
materials, such as GOx, HRP, and DAB, were dropped on the sample reservoir and
allowed the sensor to dry further at room temperature. Lastly, the PBST
solution was sent to the sample reservoir until it dried at room temperature.
Figure 1
Paper-Based Glucose Sensor Fabrication Method (a) and Dropping Process on
Glucose Sensor (b)
2.2.4. Measurements with Various Concentrations of Glucose, Artificial
Sweat, and Artificial Saliva
Various glucose concentrations in distilled
water at concentrations of 0
and
were prepared. Furthermore, the glucose solution was
dropped onto different sensor reservoirs. While experimenting, the results of
each reaction were recorded. Afterward, artificial sweat was prepared with 20
g/L NaCl, 17.5 g/L NH4Cl, 5 g/L acetic acids, and 15 g/L lactic
acid. Furthermore, 0.1 mM NaOH solution was added to the artificial sweat
solution to make the sweat produce a pH of 6.0 (Xiao et al., 2019a). The artificial saliva with 36 grams of NaCl, 1.6 grams of KCl, 0.96
grams of CaCl2, 0.8 grams of NaHCO3, and 400 ccs of
equilibrated water was made using HCl until it reached a pH of 7 (Ramadhani,
Sulistiyani, and Hidayati, 2017). Then,
pure glucose was added to the artificial sweat and saliva in various
concentrations. Accordingly, artificial sweat and saliva with various glucose
concentrations were then dropped into different sample reservoirs. With that,
the sensors were incubated at room temperature for 20 minutes, then the sample
pictures of the color were taken to get the RGB value. The RGB results of each
reaction were recorded to be validated with UV-Vis Spectrophotometry as an
additional test. Before the test, the reagents and samples were mixed with PBS
and dropped into a micro cuvette. Lastly, the absorbance results of each
example were recorded.
2.2.5. Evaluation of the Experimental Results
The RGB value indicated the color intensity that appeared due to the glucose concentration in the sample. The RGB value obtained from each glucose sensor was described in a comparison graph. From these data, the concentration and volume of reagents that provided the most optimal measurement of glucose levels were determined. The wax stamping size was then also selected to obtain the optimal reagents and the samples of the artificial sweat and saliva.
3.1. The Relation Between Reagent Concentration and
Color Change Intensity
There were three variations of
concentrations for each of the reagents used in this test. GOx and HRP had
concentrations of Meanwhile, 3,3?-diaminobenzidine (DAB) had
concentrations of
The test of various concentrations of GOx showed no
significant relationship between various concentrations of GOx and the
intensity of color change. However, GOx concentrations could affect the speed
of the color change. The higher the GOx concentration, the faster the color
change occurred. This finding was in line with the function of the enzyme
glucose oxidase as a catalyst for the oxidation of glucose to gluconic acid and
hydrogen peroxide (Yee et al., 2019; Lee et al., 2018).
Therefore, the GOx concentration of
was the concentration that could cause a fast color
change.
3.2. Stamping Wax Size was Required to Get the Optimal
Volume of Reagents and Samples
This study tested three different wax stamping diameter sizes, which were 5.5 mm, 8 mm, and 10 mm, using the RGB method. Based on the results of RGB value analysis, the most optimal wax stamping diameter was 5.5 mm. However, in this study, the wax stamping size with a diameter of 5.5 mm did not accommodate the entire volume of reagents and samples as it caused leakage. Therefore, the diameter of the wax stamping optimal for use in this study was 8 mm. The findings of this study were consistent with previous studies that predicted dimensions could affect the results in paper-based microfluidic devices. According to the study results, the error on paper-based sensor devices with a circular area of ??more than 25 mm2 was less than 10%, while the device error was 20% with a circular area of ??more than 15 mm2 (Catalan-Cario et al., 2020). The area of ??a circle used in this sensor was 50.24 mm2 (circle diameter = 8 mm). With this area of the circle, the total error was less than 10% and would be considered an optimal wax stamping size.
According to another study, Santana-Jimenez et
al. used wax stamping with a diameter of 3 mm. With this diameter, the
amount of enzyme solution, reagent, and sample dripped was 5?L (Santana-Jimenez et al.,
2018), and the area of the wax stamping was ??7 mm2.
Therefore, the ratio between the amount of dripped volume and the circle area (7 mm2) in the
Santana-Jimenez study was 1:1.4. In this study, the optimal diameter was 8 mm
with a circular area of ??50 mm2. Thus, we obtained a comparison
between the amount of volume that dripped
and the area of the circle (50 mm2) in this
research was approximately 1:1.4. From the results of these calculations, the
diameter size of this study was in accordance with the diameter of the
reference journal.
3.3. The Correlation of Glucose Concentration in
Samples and Intensity of Color Change
3.1.1. Pure
Glucose
3.3.2. Artificial
Sweat
This study utilized 11 glucose
concentration variations in artificial sweat. It was the same as in the pure
glucose test. The test results using the RGB method showed a strong negative
correlation between the glucose concentration in artificial sweat and the three
RGB values. The researchers succeeded in proving this correlation with the
Spearman correlation coefficient of -0.9931 (R2 = 0.9862) for RGB-R,
and the Pearson correlation coefficient, respectively, for RGB-G and RGB-B were
-0.9469 and -0.9048 (R2 = 0.8966 and 0.8187). The linear regression
equation of the RGB-R data calibration curve is y = -0.011x + 189.664 (figure
3a). The equation was made based on RGB-R data because the RGB-R values
??provided the most visible difference and significantly contributed to color
changes caused by enzyme catalytic reactions (Xiao et al., 2019a). The findings in this study were in accordance
with the findings by Xiao et al., who found something similar, which had
a negative linear relationship between the RGB-R values ??and 0 to 1500 uM
glucose concentrations in artificial sweat. Therefore, paper-based sensors can
be relied upon to detect glucose in sweat (Xiao et al., 2019a).
The glucose level in sweat is 10-1110 ?M (Xiao et al., 2019b). The sweat glucose level of normal people is 60-110 ?M, while the sweat glucose level of diabetic patients is 10-1000 ?M (Bruen et al., 2017). The glucose sensor in this study could detect glucose levels in artificial sweat with a concentration of 0 to 3000 ?M. Therefore, it could detect sweat glucose levels in normal people to diabetic patients. In addition, the difference in color intensity could be seen visually. The higher the glucose concentration in artificial sweat, the more pronounced the brown color on the glucose sensor (Figure 2). This color changed because the glucose in the artificial sweat samples activated a reagent-chromogen system reaction that resulted in an increased color intensity along with an increase in glucose concentration. Based on these findings, the strong correlation between glucose concentration in artificial sweat and color intensity could be the basis for the design concept of a glucose sensor using paper-based colorimetric methods.
Figure 2 Color Differences in Four Different Concentrations of Glucose in Artificial Sweat
Figure 3 Linear Regression Graph of
Artificial Sweat RGB Test (a) and Linear Regression Graph of Artificial Sweat
Spectrophotometric UV-Vis Test (b)
The
last step of this research was validating the RGB test results using the UV-Vis
Spectrophotometry method. First, we determined the appropriate wavelength of
the reaction between the reagent-chromogen and glucose in the sample. The peak
absorbance value of the reaction was at a wavelength of 419 nm. Then, we
proceeded with testing artificial sweat samples with five glucose
concentration variations (0, 300 ?M, 1500 ?M, 2400 ?M, and 3000 ?M) at a
wavelength of 419 nm. Based on the results, there was a strong positive
correlation between glucose concentration in artificial sweat and light
absorbance at 419 nm with a Spearman correlation coefficient of 0.993 (R2
= 0.985) and a linear regression equation y = 0.00006x + 0.025 (Figure 3b). The
positive correlation explains that the higher the glucose concentration in
artificial sweat, the higher the light absorbance value at a wavelength of 419
nm. In comparison, the negative correlation in the RGB equation illustrates
that the higher the glucose concentration, the lower the RGB value. This study
proved that the RGB method was valid. Therefore, the RGB approach can be
applied to the design concept of detecting glucose in sweating paper-based
colorimetry.
3.3.3. Artificial Saliva
In the test of artificial saliva samples
with RGB, the researcher made the saliva glucose concentration in 11
concentration variations as in the pure glucose test. Based on the experimental
results, a strong correlation was found between various glucose concentrations
in artificial saliva and the intensity of the color change. The higher the sample
concentrations, the higher the intensity of the color formed. In previous
research from Xiao et al., the linear regression equation y = -0.19x + 231.68
was obtained with a value of R2 = 0.9896. This value indicated that
the R (red) value provided a good linear relationship (Xiao et al., 2019a). In this study, we
obtained a linear regression equation from the calibration curve of R(red) data
on RGB, namely y = -0.015x + 192.686, with a value of R2 = 0.991 (Figure 5a). This value indicates a good
correlation between the intensity of the color produced and various glucose
concentrations in artificial saliva. This correlation was even better than the
previous research conducted by reference journals.
The high correlation proves that the
prototype can significantly measure saliva glucose concentrations from 0-3000
?M. The average saliva concentration in normal people is 8-210 ?M. While in
diabetes patients, the saliva glucose concentration was 100-700 ?M (Siddiqui et al., 2018). Therefore, this
prototype can detect salivary glucose levels in people with diabetes. Color
gradations from the artificial saliva glucose test results with concentrations
of can also be seen with the naked eye (Figure
4).
Figure 4 Color Differences in Four Different Concentrations
of Glucose in Artificial Saliva
After testing with RGB,
UV-Vis spectrophotometry was used to validate all the results obtained using an
artificial saliva sample that consisted of 5 glucose concentration variations consisted of 5 glucose concentration variations (0,
300 ?M, 1500 ?M, 2400 ?M, and 3000 ?M). With a wavelength of 419 nm, the trial
results showed a strong correlation between the various glucose concentrations
in the artificial saliva and the resulting color intensity. All data from this
experiment were made in the form of a calibration curve and obtained a linear
regression equation y = 0.0006x + 0.025 with a value of R2 = 0.9831
(Figure 5b). This value indicated a good correlation between the absorbance value
??and the various glucose concentrations inartificial saliva samples. Similar to the study on sweat, the results of the data obtained in the
assay with RGB and UV-Vis spectrophotometry gave inversely correlated results.
The higher the glucose concentration, the lower the RGB value, while the
absorbance value of the UV-Vis spectrophotometric test was lower. The results of this trial have proven to validate the results
obtained in research with RGB.
Figure 5 Linear
Regression Graph of the RGB Test of Artificial Saliva (a) and Linear Regression
Graph of the UV-Vis Spectrophotometric Test of Artificial Saliva (b)
3.3.4. Sensor
Sensitivity and Accuracy
The smartphone device used
in this study would help glucose detection be more practical. Therefore, there
are several things to note. First, taking pictures will be different if you do
not follow the black box instructions correctly. In addition, the RGB value in
the application will change if the user uses the zoom-in/out feature, which
will reduce the validity of reading the glucose concentration results in the
sample. Therefore, adjustments are needed to the black box to help provide
similar image results for each type of smartphone used.
In addition to using a
black box, wax stamping could also affect the sensitivity and accuracy of the
glucose sensor. The first was about the successful penetration of paraffin on
the filter paper. In our research, the paraffin occasionally did not penetrate
the back of the filter paper, reducing the sensitivity and accuracy of the
results. The second was the possibility of paraffin entering the sample
reservoir circle during the wax stamping process. Paraffin droplets that
accidentally dropped into the sample reservoir could also reduce the accuracy
of the glucose sensor. Therefore, it is more advisable to use a more guaranteed
method in terms of shape and print results, such as wax printing or beeswax printing which are useful for biological detection (Nunut et
al., 2020).
This
study proved a highly correlated relationship between the concentration of
Horseradish Peroxidase (HRP) as an enzyme and 3,3'-diaminobenzidine (DAB) as a
chromogen with the color change intensity by the colorimetric method. Glucose
Oxidase (GOx) affected the speed of color changes on the glucose sensor. The
size of the hydrophobic barrier can also affect the intensity of the resulting
color. The size of the wax stamping that gave optimal results had a ratio of
1:1.4 between the area of the circle formed by wax stamping and the volume of
liquid that was dropped. In addition, this study proved a strong correlation
between various glucose concentrations and color intensity. The solutions used
in this research consist of variations of pure glucose solutions, artificial
sweat, and saliva solutions concentrations. R2 values proved a
strong correlation, 0.9928 for pure glucose, 0.9862 for glucose in artificial
sweat, and 0.991 for artificial saliva. The glucose sensor in this study has
been proven to measure glucose levels with a concentration of 0 to optimally, including normal glucose levels and glucose levels in diabetic
patients in sweat and saliva. This finding could be the basis for developing a
non-invasive glucose level detection device in sweat and saliva.
This research was funded by the Indonesian Ministry
of Research and Technology/National Research and Innovation Body in 2021
(contract no. 1208/LL3/PG/2021).
Altundemir, S., Uguz,
A.K., Ulgen, K., 2017. A Review on Wax Printed Microfluidic Paper-Based Devices
for International Health. Biomicrofluidics, Volume 11(4), p. 041501-23
Bruen, D., Delaney,
C., Florea, L., Diamond, D., 2017. Glucose Sensing for Diabetes Monitoring:
Recent Developments. Sensors, Volume 17(8),
p. 1866
Catalan-Carrio, R.,
Akyazi, T., Basabe-Desmonts, L., Benito-Lopez, F., 2020. Predicting Dimensions
in Microfluidic Paper Based Analytical Devices. Sensors, 21(1), p. 101
Dewi,
D.S., Irfoni, A.R., Rahman, A., 2017. Kansei Engineering Approach for Designing
a Self-Monitoring Blood Glucose Application. International Journal of
Technology, Volume 8(2), pp. 272–282
Dominguez,
R.B., Orozco, M.A., Chavez, G., Marquez-Lucero, A., 2017. The Evaluation of a
Low-Cost Colorimeter for Glucose Detection in Salivary Samples. Sensors, Volume
17(11), pp. 2495
Ekawita, R., Nasution,
A.A., Yuliza, E., Suardi, N., Suwarsono, 2020. Development of Non-Invasive
Blood Glucose Level Monitoring System using Phone as a Patient Data Storage. Jurnal
Penelitian Fisika dan Aplikasinya (JPFA), Volume 10(2), pp. 103–113
Gabriel, E.F.M.,
Garcia, P.T., Lopes, F.M., Coltro, W.K.T., 2017. Paper-Based Colorimetric
Biosensor for Tear Glucose Measurements. Micromachines, Volume 8(4), p.
104
Gordon, C, 2019.
Blood Glucose Monitoring in Diabetes: Rationale and Procedure. British
Journal of Nursing, Volume 28(7), pp. 434–439
Gupta, S., Nayak, M.,
Sunitha, J., Dawar, G., Sinha, N., Rallan, N.S., 2017. Correlation Of Salivary
Glucose Level With Blood Glucose Level In Diabetes Mellitus. Journal of Oral
and Maxillofacial Pathology, Volume 21(3), p. 334
He, Y., Wu, Y., Fu, J.-Z.,
Wu, W.-B., 2015. Fabrication of Paper-Based Microfluidic Analysis Devices: a
Review. RSC Advances, Volume 5(95), pp. 78109–78127
International
Diabetes Federation (IDF), 2019. IDF Diabetes Atlas. 9th edition.
Available online at: https://www.diabetesatlas.org
Jangi, A.R.H., Jangi, M.R.H., Jangi, S.R.H. 2020. Detection
Mechanism and Classification Of Design Principles of Peroxidase Mimic Based
Colorimetric Sensors: A Brief Overview. Chinese Journal of Chemical
Engineering, Volume 28(6) pp. 1492–1503
Jangi, A.R.H., Jangi,
M.R.H., Jangi, S.R.H., 2020. Detection Mechanism and Classification of Design
Principles of Peroxidase Mimic Based Colorimetric Sensors. Chinese Journal
of Chemical Engineering, Volume 28(6), pp. 1492–1503
Jin, S., Liu, L., Fan,
M., Jia, Y., Zhou, P., 2020. A Facile Strategy for Immobilizing GOD and HRP
Onto Pollen Grain and Its Application to Visual Detection of Glucose. International
Journal of Molecular Sciences, Volume 21(24), p. 9529
Karpova, E.V.,
Shcherbacheva, E.V., Galushin, A.A., Vokhmyanina, D.V., Karyakina, E.E.,
Karyakin, A.A., 2019. Noninvasive Diabetes Monitoring through Continuous
Analysis of Sweat Using Flow-Through Glucose Biosensor. Analytical
Chemistry, Volume 91(6), p. 3778
Lee, H., Hong, Y.J.,
Baik, S., Hyeon, T., Kim, D.-H., 2018. Enzyme-Based Glucose Sensor: From
Invasive to Wearable Device. Advanced
Healthcare Materials, Volume 7(8), pp. 1701150
Liu, S., Su, W., Ding.
X., 2016. A Review on Microfluidic Paper-Based Analytical Devices for Glucose
Detection. Sensors. Volume 16(12) p. 2086
Lookmatix, 2020. Color
Grab™ [Mobile app]. Google Play. Available online at: https://play.google.com/store/apps/details?id=com.loomatix.colorgrab&hl=id
Meetoo, D., Wong, L.,
Fatani, T., 2018. 'Knowing Where I am': Self-Monitoring of Blood Glucose in
Diabetes. British Journal of Nursing, Volume 27(10), pp. 537–541
Nadaf, A., Hassan,
Z., Farooq, S., 2017. Salivary Glucose Level and its Correlation with Blood
Glucose Level in Patients with Diabetes Mellitus-an in-Vivo Study. International
journal of Contemporary Medical Research, Volume 4(12), pp. 17–20
Nunut,
I., Whulanza, Y., Kassegne, S., 2020. Testing of Beeswax Printing Technology in
the Design of a Paper-Based Microfluidic System. International Journal of
Technology, Volume 11(5), pp. 1036–1045
Perdani, M.S.,
Juliansyah, M.D., Putri, D.N., Utami, T.S., Hudaya, C., Yohda, M., Hermansyah,
H., 2020. Immobilization of Cholesterol Oxidase in Chitosan Magnetite Material
for Biosensor Application. International Journal of Technology, Volume 11(4),
pp. 754–763
Ramadhan, A.,
Sulistiyani, S., Hidayati, L., 2017. Uji Kedalaman Porositas Permukaan Bahan
Pit dan Fissure Sealant Berbasis Resin Komposit dan Ionomer Kaca Setelah
Direndam Saliva Buatan pH 5,5 (Depth Porosity Test of Surface Materials Pit
and Fissure Sealant Based Resin Composite and Glass Ionomer After). Pustaka Kesehatan, Volume 5(3), pp. 454–460
Santana-Jiménez L.,
Márquez-Lucero A., Osuna V., Estrada-Moreno I., Dominguez R., 2018. Naked-Eye
Detection of Glucose in Saliva with Bienzymatic Paper-Based Sensor. Sensors, Volume 18(4), p. 1071
Siddiqui, S., Zhang,
Y., Lloret, J., Song, H., Obradovic, Z., 2018. Pain-Free Blood Glucose
Monitoring Using Wearable Sensors: Recent Advancements and Future Prospects. IEEE
Reviews in Biomedical Engineering, Volume 11, pp. 21–35
Singh, A., Lantigua,
D., Meka, A., Taking, S., Pandher, M., Camci-Unal, G., 2018. Paper-Based
Sensors: Emerging Themes and Applications. Sensors,
Volume 18(9), pp. 2838
Soum, V., Park, S.,
Brilian, A.I., Kwon, O.-S., Shin, K., 2019. Programmable Paper-Based
Microfluidic Devices for Biomarker Detections. Micromachines, Volume10(8), p. 516
Vaquer, A., Barón,
E., Rica, R., 2021. Detection of Low Glucose Levels in Sweat with Colorimetric
Wearable Biosensors. The Analyst, Volume 146(10), p. 3273
Wang, T., Guo, K.,
Hu, X., Liang, J., Li, X., Zhang, Z., Xie, J., 2020. Label-Free Colorimetric
Detection of Urine Glucose Based on Color Fading Using Smartphone Ambient-Light
Sensor. Chemosensors, Volume 8(1), p. 10
Wei, X., Tian, T.,
Jia, S., Zhu, Z., Ma, Y., Sun, J.-J., Lin, Z., Yang, C.J., 2016. Microfluidic
Distance Readout Sweet Hydrogel Integrated Paper-Based Analytical Device
(µDiSH-PAD) for Visual Quantitative Point-of-Care Testing. Analytical Chemistry, Volume 88(4), pp. 2345–2352
Xiao, G., He, J.,
Chen, X., Qiao, Y., Wang, F., Xia, Q., Yu, L., Lu, Z, 2019a. A Wearable, Cotton
Thread/Paper-Based Microfluidic Device Coupled with Smartphone for Sweat
Glucose Sensing. Cellulose, Volume 26(7),
pp. 4553–4562
Xiao, J., Liu, Y., Su,
L., Zhao, D., Zhao, L., Zhang, X., 2019b. Microfluidic Chip-Based Wearable
Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose. Analytical Chemistry, Volume 91(23), pp.
14803–14807
Xiong, Y., Pei, K.,
Wu, Y., Xiong, Y., 2017. Colorimetric ELISA Based on Glucose Oxidase- Regulated
The Color of Acid–Base Indicator For Sensitive Detection of Aflatoxin B1 in
Corn Samples. Food Control, Volume 78(1),
pp. 317–323
Yee, Y., Hashim, R.,
Mohd, A., Bustami, Y., 2019. Colorimetric Analysis of Glucose Oxidase-Magnetic
Cellulose Nanocrystals (CNCs) for Glucose Detection. Sensors, Volume 19(11)
p. 2511
Zafar, H., Channa,
A., Jeoti, V., Stojanovi?, M., 2022. Comprehensive Review on Wearable
Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors, Volume
22(2), p. 638
Zheng, H., He, J., Li, P., Guo, M., Jin, H., Shen, J., Xie, Z., Chi, C.,
2018. Glucose Screening Measurements and Non-invasive Glucose Monitor Methods. Procedia
Computer Science, Volume 139, pp. 613–621
