Characterization and Application of TiO2/ZnO Nanoparticles as Pigments in Matt-Type Water-Based Paint
Corresponding email: novesarjamarun@sci.unand.ac.id
Published at : 04 Apr 2023
Volume : IJtech
Vol 14, No 2 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i2.5501
Isfa, B., Jamarun, N., Emriadi, Arief, S., Ritonga, A.H., Tanjung, D.A., Sisca, V., 2023. Characterization and Application of TiO2/ZnO Nanoparticles as Pigments in Matt-Type Water-Based Paint. International Journal of Technology. Volume 14(2), pp. 320-329
| Boy Isfa | Department of Chemistry, Universitas Andalas, Padang-25163, Indonesia |
| Novesar Jamarun | Department of Chemistry, Universitas Andalas, Padang-25163, Indonesia |
| Emriadi | Department of Chemistry, Universitas Andalas, Padang-25163, Indonesia |
| Syukri Arief | Department of Chemistry, Universitas Andalas, Padang-25163, Indonesia |
| Ahmad Hafizullah Ritonga | Institut Kesehatan Medistra Lubuk Pakam, Deli Serdang-20512, Indonesia |
| Denny Akbar Tanjung | Faculty of Pharmacy, Institut Kesehatan Deli Husada Deli Tua, Deli Serdang-20355, Indonesia |
| Vivi Sisca | Department of Biology Education, Universitas Merangin, Jambi-37313, Indonesia |
This study
investigates the characteristics of TiO2/ZnO nanoparticles and the
application of TiO2/ZnO in matt-type water-based paint as a pigment.
This study aims to determine the quality of TiO2-ZnO pigments in
water-based paints in terms of whiteness, hiding power, dispersibility, and
gloss. Matt-type water-based paint has been made by mixing water and additives
substances in a high-speed mixer to result in a mill base paste, which is mixed
with the pigment of TiO2/ZnO (25:75), CaCO3 filler,
water, and additives, then filtered and mixed again with the C-817 binder. The
characterization results showed that TiO2/ZnO nanoparticles
contained TiO2 of 18.62% and ZnO of 77.49%, with an average particle
diameter of 151.54 nm. TiO2/ZnO has a crystal size of 28.4 nm and a
crystallinity degree of 72.3%. The application of TiO2/ZnO
nanoparticles as a pigment in matt-type water-based paint resulted in good
dispersion and hiding power and achieved whiteness of 82.2 and gloss at 60° of
2.00 better than TiO2 of 81.8 and gloss at 60° of 1.77.
Matt-type water-based paint; Pigment; TiO2; TiO2/ZnO; ZnO
Paint is a
complex mixture consisting of resins, pigments, solvents, fillers, and other
additives used to coat the surface of a material to beautify, strengthen, or
protect the material. There are two types of paint, namely water-based paint
and solvent-based paint. Water-based paint commonly used is the matt type which
gives an even and non-reflective finish. This matt water-based paint is the
most popular choice because it is easy to apply on smooth interior surfaces.
The matt-type application is generally used on ceilings and walls with an even
finish and a sheen level of less than 10%, giving it a non-reflective
appearance (Chen et al., 2022; Peruchi et al., 2021).
In the paint industry, the main white pigment popularly used is titanium dioxide (TiO2) because of its whiteness, high coverage, refractive index, covering power, achromatic force, good dispersion, and resistance to ultraviolet (UV) rays (Islam et al., 2020; Karakas and Çelik, 2018). These advantages have caused TiO2 to be widely developed and researched and become the main choice in its application as a pigment compared to zinc oxide (ZnO), lithopone, and others (Gao et al., 2022; Costa et al., 2017).
The TiO2 cannot be obtained naturally but is extracted from ilmenite ore. TiO2 can also be synthesized using the sol-gel method (Solanki et al., 2021; Yuwono et al., 2014), sonochemical (Rosales et al., 2021), hydrothermal, solvothermal (Mamaghani, Haghighat, Lee, 2019; Sofyan et al., 2018, 2019), microwave (Li et al., 2021), co-precipitation (Bhogaita and Devaprakasam, 2021), and direct oxidation (Daraee et al., 2018). However, the synthesis of TiO2 is limited by the environmental pollution generated by industrial processes, the shortage of titanium resources, and the high selling price. Therefore, it is necessary to develop TiO2 by mixing it with other white pigments with better or almost similar quality to reduce the high demand for TiO2 nanoparticles (Razali et al., 2022; Isfa et al., 2022; Dell’Edera et al., 2021; George et al., 2021).
Zinc oxide (ZnO) is the main white pigment that
has long been used in the organic paint industry because of its environmentally
friendly nature. ZnO is naturally found in the form of the mineral
zincite. The advantages of ZnO as a pigment are that it is
stable against UV rays (does not change color), long lasts on both water-based
and oil-based paints, and increases color retention. Compared with the other
white pigments, ZnO has the least tendency to turn yellow (Papp et
al., 2022; Ma et
al., 2019; Adiwibowo, Ibadurrohman, Slamet, 2018). Based on the advantages of ZnO as a
pigment, it is necessary to develop research related to ZnO combined with TiO2
to result in TiO2/ZnO nanoparticles and implement the use of
matt-type water-based paints. The novelty in this work is related to the
application of TiO2/ZnO mixtures as a pigment in matt-type
water-based paints.
A few previous studies related to the
development of TiO2/ZnO nanoparticles have been reported, including Miklecic et al.
(2015), which studied the effect of TiO2/ZnO nanoparticles on the
properties of waterborne polyacrylate coatings in outdoor conditions, where the
existence of ZnO nanoparticles reduced coating flow time, increased pH, and
decreased elongation. In contrast, the TiO2 increased the glass
transition so that the combination of TiO2/ZnO increased color
stability. El-Kader et al.
(2021) observed morphological, structural, and antibacterial on TiO2/ZnO
(50:50) nanocomposites from Hibiscus rosa-senensis extract, which
produced an increase in the crystal size growth of ZnO with uneven distribution
and irregular particle shape. It is different with the crystal size growth of
TiO2 was inhibited the distribution particles and shape were evenly
and uniform. Baudys et al.
(2015) conducted a weathering test and photocatalytic activity on
self-cleaning acrylic paint based on ZnO and TiO2, where the results
showed that the paint sample TiO2-based pigment increased
photoactivity during exposition on the QUV panel, while the paint sample
ZnO-based ones showed a high initial photocatalytic activity but decreased
during exposition in the QUV panel. Lv et al.
(2019) studied radiation stability in solar reflective coatings using TiO2
and ZnO pigments, where the particle size affects the stability of the paint
coating from radiation. Using TiO2/ZnO mixture as
pigments in acrylic paints has achieved better photocatalytic activity than
pure TiO2 and ZnO (Song et
al., 2021; Jašková, Hochmannová, Vytrasova, 2013). The objective of this study is to characterize TiO2/ZnO
nanoparticles and observe the quality improvement of TiO2/ZnO
nanoparticles applied as pigments in matt-type water-based paints.
2.1. Materials
Titanium dioxide rutile
(SR-2377) was obtained from Shandong Dongjia Group Co., Ltd. Shandong, China.
Zinc oxide (ZnO) was supplied by Evergreen Chemical Factory Co., Ltd. Shaanxi,
China. Calcium carbonate (Omyacarb-6 GD) as a filler was obtained from PT. Camco
Omya Indonesia, Jakarta, Indonesia.
The styrene-acrylic binder
(C-817) and super-plasticizer (SPC) were received from PT. Inawan Chemtex
Sukses Abadi, Jakarta, Indonesia. The optical brightener agent (OBA) was
supplied by PT. Graha Jaya Chemical, Jakarta, Indonesia. Dispersing agents,
anti-foaming agents, wetting agents, coalescent agents, antimicrobial agents
(biocide), thickening agents, and ethylene glycol are used for commercial
water-based paint recipes.
2.2. Nanoparticles
Characterization
The chemical composition of the pigments
(TiO2, ZnO, and TiO2-ZnO) was analyzed by X-Ray
Fluorescence (XRF) - PANalytical Epsilon 3. The pigments' crystal structure,
size, and crystallinity degree were determined by X-Ray Diffraction (XRD) -
PANalitycal X'Pert PRO MPD. Morphological of the pigments were characterized by
Field Emission - Scanning Electron Microscope (FE-SEM) - FEI Inspect F50. The
TiO2-ZnO (25:75) nanoparticle pigments characterized were made from
a mixing process using a planetary mixer (KNS-60 LB).
2.3. Manufacturing Process of Matt-Type Water-Based Paint
Water, thickening agent,
wetting agent, dispersing agent, antimicrobial agent, anti-foaming agent, and
ethylene glycol were added to a high-speed mixer according to Table 1, stirred using a zirconium ball mill at 2000 rpm for
1 hour, filtered with a sieve (mesh 200) and allowed to cool at room
temperature. The results obtained are in the form of mill-base paste. The mill
base paste, pigment (TiO2, ZnO, or TiO2-ZnO), optical
brightener agent, super-plasticizer, CaCO3 filler, water, and
coalescing agent were added into a high-speed mixer according to Table 1,
stirred at 800 rpm for 30 min, filtered with a sieve (mesh 200), and
dispersibility of the pigment was observed. Next, the C-817 binder was added
and stirred at 100 rpm for 15 min. The results obtained are matt-type
water-based paint with various pigments, referred to as a P sample (Karakas and Celik, 2018; Somtürk et al., 2016).
Figure 1 Preparation
and application of TiO2/ZnO in matt-type water-based paint
2.4. Application of Nanoparticles in Matt-Type Water-Based
Paint
Matt-type water-based paint
is applied to the surface of the panel paper using a rod coating (100),
allowed for 24 h, and the obtained result was visually observed the hiding
power in panel paper. Next, whiteness and gloss were measured using a whiteness
meter (BGD 585) and a gloss meter (BGD 516).
3.1. XRF Analysis of Pigments
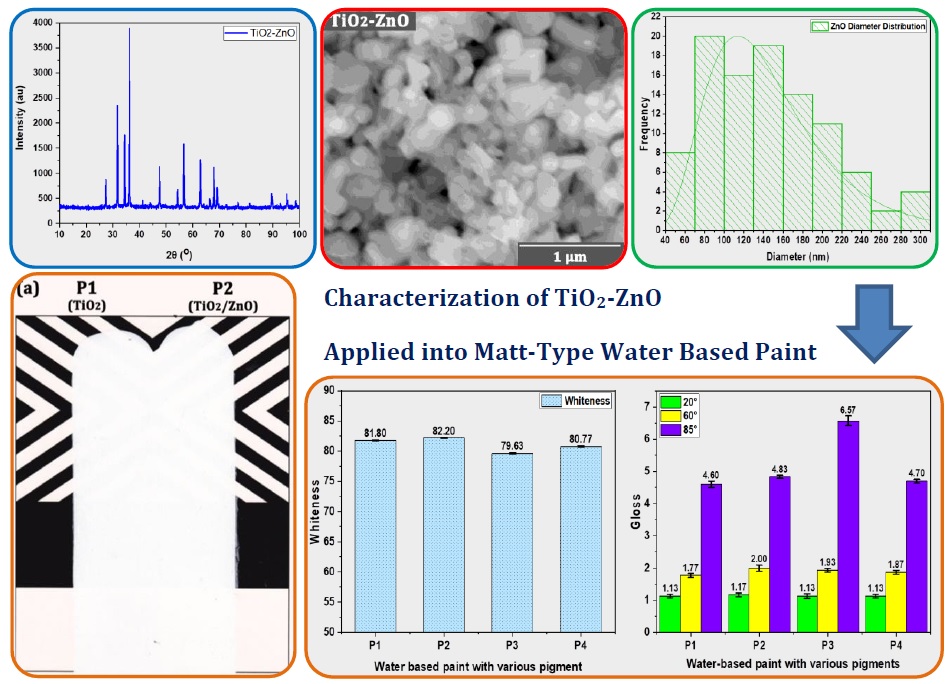
The results of XRF analysis on TiO2, ZnO, and TiO2/ZnO nanoparticles (Table 2) displayed that rutile had a TiO2 concentration of 94.57%. Zinc oxide nanoparticles had a ZnO concentration of 96.11%. In the TiO2-ZnO mixture, the concentration of TiO2 is 18.62%, and ZnO is 77.49%. These results indicate that the mixing of TiO2/ZnO with a composition of 25:75 showed no significant difference with the concentration of TiO2/ZnO in the mixture, which means that there is no chemical interaction. The concentration of TiO2 rutile was not significantly different from previous research (Sisca et al., 2021; Miklecic et al., 2015).
Table 2 Chemical Composition of TiO2, TiO2/ZnO, and ZnO
The
crystallinity degree, size, and shape crystal of TiO2, TiO2/ZnO,
and ZnO were analyzed by XRD. The XRD pattern is displayed in Figure 2. The
characteristic peaks of TiO2 at 2 are 27.43°, 36.08°, 39.21°,
41.26°, 44.09°, 54.36°, 56.67°, 62.81°, 64.11°, 69.06°, 69.86°, 82.40°, and
89.63°. The peaks are suitable to the characteristic peak of rutile
(01-087-0290), which has a crystallinity degree of 24.0 % and a crystal size of
29.8 nm with a tetragonal shape. This result also corresponds to previous
reports (Razali et al., 2022; Daniyal, Akhtar, Azam, 2019).
The
characteristic peaks of TiO2/ZnO at 2? are 27.41°, 31.74°, 34.40°,
36.23°, 44.00°, 47.54°, 54.33°, 56.61°, 62.87°, 66.40°, 67.99°, 69.10°, 72.47°,
76.99°, 89.62°, 92.71°, 95.34°, and 98.57°. The peaks are suitable to the
characteristic peak of TiO2 rutile (01-072-4814) and zincite
(03-065-3411), which has a crystallinity degree of 72.3 %, a crystal size of
28.4 nm with tetragonal and hexagonal shapes. This result corresponds to
crystals of TiO2/ZnO (El-Kader et al., 2021; Mazabuel-Collazos, Gómez, Rodríguez-Páez, 2019).
The
characteristic peaks of ZnO at 2are 31.70°, 34.36°, 36.19°, 47.49°, 56.56°,
62.83°, 66.37°, 67.92°, 69.05°, 72.58°, 76.90°, 81.43°, 89.61°, 92.65°, 95.24°,
and 98.55°. The peaks are suitable to the characteristic peak of zincite
(01-075-7917), which has a crystallinity degree of 64.1 % and a crystal size of
25.4 nm with a hexagonal shape. This result corresponds to previous reports (El-Kader et al.,
2021).
Figure 2 XRD of (a) TiO2; (b) TiO2/ZnO; and (c) ZnO
3.3. SEM
Analysis of Pigments
The morphological analysis results of nanoparticles
using FE-SEM with a magnification of 10,000x (10 µm) and 100,000x (1 µm) (Figure 3) has displayed that all pigments have very small
sizes (<200 nm), smooth, and uniform shapes (Mazabuel-Collazos, Gómez, Rodríguez-Páez, 2019; Karakas and Çelik, 2018; Mikle?i? et al., 2015).
Figure 4 Diameter distribution of (a) TiO2;
(b) TiO2/ZnO, and (c) ZnO
3.4. Application of Nanoparticles as Pigments in Matt-Type
Water-Based Paint Against Dispersibility, Hiding Power, Whiteness, and Gloss
|
Recipe Name |
Whiteness |
Gloss |
Hiding Power |
Dispersibility | ||
|
20° (95.1) |
60° (96.3) |
85° (99.7) | ||||
|
P1 |
81.80 + 0.10 |
1.13 + 0.06 |
1.77 + 0.06 |
4.60 + 0.10 |
Good* |
Good** |
|
P2 |
82.20 + 0.10 |
1.17 + 0.06 |
2.00 + 0.10 |
4.83 + 0.05 |
Good* |
Good** |
|
P3 |
79.63 + 0.15 |
1.13 + 0.07 |
1.93 + 0.06 |
6.57 + 0.15 |
Good* |
Good** |
|
P4 |
80.77 + 0.15 |
1.13 + 0.06 |
1.87 + 0.06 |
4.70 + 0.06 |
Medium* |
Good** |
Note:
* Evaluation
of hiding power results is carried out based on visual observations of the
paint coating on panel paper, which refers to the standard color of white and black.
** Evaluation of pigment dispersibility was carried out based on the number of nanoparticles left on the sieve (mesh 200), which were observed visually.
Figure 5 A Hiding Power of (a) P1 (TiO2) & P2 (TiO2/ZnO);
(b) P1 (TiO2) & P3 (TiO2/ZnO/OBA/SPC); and (c) P1
(TiO2) & P4 (ZnO/OBA)
Figure 6 (a) Whiteness and (b) Gloss of Water-Based Paint
with Various Pigments
Reflection
angles of 20° and 60° in sample P2 have resulted in better gloss values ??than
other samples (Figure 6b), which is
influenced by the high crystallinity degree of TiO2/ZnO
nanoparticles. Meanwhile, at a reflection angle of 85°, sample P3 has resulted
in a significant increase in the gloss value of 6.57, which was influenced by
the presence of OBA and SPC in sample P3.
In terms of
dispersibility, hiding power, whiteness, and gloss, it is known that sample P1
using TiO2/ZnO nanoparticles as a pigment has better quality than
sample P1 when applied to matt-type water-based paint. The amount of pigment in
water-based paint formulas commonly for the matt-type is 7-10%, semi-gloss is
20-25%, and gloss is above 35%. With a gloss value below 10%, the water-based
paint was categorized as a matt-type.
The TiO2/ZnO nanoparticles have been successfully combined with
the composition (25:75), which resulted in a crystallinity degree of 72.3%, a
crystal size of 28.4 nm with tetragonal and hexagonal shapes. These
nanoparticles contain 18.62% TiO2 and 77.49% ZnO compounds, with an
average particle diameter of 151.54 nm. TiO2/ZnO nanoparticles also
have been successfully applied as pigment to a matt-type water-based paint
which achieved good dispersibility and hiding power and resulted in a whiteness
of 0.48% and a gloss at 60° of 12.99% were better than TiO2. In the
future, a TiO2/ZnO mixture will be made using a chemical method
applied to a matt-type water-based paint with a composition according to the
current works.
The author would like to thank PT.
Inawan Chemtex Sukses Abadi for the opportunity to conduct research and use
the facilities in the Technical Department Laboratory.
Adiwibowo, M.T., Ibadurrohman, M., Slamet,
2018. Synthesis of ZnO nanoparticles and their nanofluid stability in the presence of a palm
oil-based primary alkyl sulphate surfactant for detergent application. International
Journal of Technology, Volume 9(2), pp. 307–316
Baudys, M., Krysa, J., Zlamal, M., Mills, A.,
2015. Weathering tests of photocatalytic facade paints containing ZnO and TiO2.
Chemical Engineering Journal, Volume 261, pp. 83–87
Bhogaita, M., Devaprakasam, D., 2021. Hybrid photoanode of TiO2-ZnO synthesized by co-precipitation route for
dye-sensitized solar cell using phyllanthus reticulatas pigment sensitizer. Solar
Energy, Volume 214, pp. 517–530
Chen, M.C., Koh, P.W., Ponnusamy, V.K., Lee,
S.L., 2022. Titanium dioxide and other nanomaterials based antimicrobial
additives in functional paints and coatings: review. Progress in Organic
Coatings, Volume 163, p. 10666
Costa, J.R.C., Correia, C., Góis, J.R.,
Silva, S.M.C., Antunes, F.E., Moniz, J., Serra, A.C., Coelho, J.F.J., 2017.
Efficient dispersion
of TiO2 using tailor made poly(acrylic acid) -based block
copolymers, and its incorporation in water based paint formulation. Progress
in Organic Coatings, Volume 104, pp. 34–42
Daniyal, M., Akhtar, S., Azam, A., 2019.
Effect of nano-TiO2
on the properties of cementitious composites under different exposure
environments. Journal of Materials Research and Technology, Volume 8(6),
pp. 6158–6172
Daraee, M., Baniadam, M., Rashidi, A.,
Maghrebi, M., 2018. Synthesis of TiO2-CNT hybrid nanocatalyst and
its application in direct oxidation of H2S to S. Chemical Physics,
Volume 511, pp. 7–19
Dell’Edera, M., Lo Porto, C., De Pasquale,
I., Petronella, F., Curri, M.L., Agostiano, A., Comparelli, R., 2021.
Photocatalytic TiO2-Based coatings for environmental applications. Catalysis
Today, Volume 380, pp. 62–83
El-Kader, M.F.H.A., Elabbasy, M., Adeboye, A.A., Zeariya, M.G.M., Menazea, A.., 2021. Morphological, structural and
antibacterial behavior of eco-friendly of ZnO/TiO2 nanocomposite
synthesized via hibiscus rosa-sinensis extract. Journal of Materials
Research and Technology, Volume 15, pp. 2213–2220
Gao, H., Yang, S., Mao, D., Long, M., Qu, X.,
2022. Significant zinc release from widely-used commercial lithopone pigments
under solar irradiation. Environmental Pollution, Volume 292, p. 118352
George, J., Gopalakrishnan, C.C., Manikuttan,
P.K., Mukesh, K., Sreenish, S., 2021. Preparation of multi-purpose TiO2
pigment with improved properties for coating applications. Powder Technology,
Volume 377, pp. 269–273
Isfa, B., Jamarun, N., Emriadi, Ritonga,
A.H., Sisca, V., Faisal, H., Tanjung, D.A., 2022. Precipitated calcium
carbonate/lithopone nanoparticles as substitution of TiO2 pigment for
matte-type water-based paint. Rasayan Journal of Chemistry, Volume
15(4), pp. 2342–2349
Islam, M.T., Dominguez, A., Turley, R.S., Kim, H., Sultana, K.A., Shuvo, M., Alvarado-Tenorio, B., Montes, M.O., Lin, Y., Gardea-Torresdey, J., Noveron, J.C., 2020. Development of photocatalytic
paint based on TiO2 and photopolymer resin for the degradation of
organic pollutants in water. Science of The Total Environment, Volume
704, p. 135406
Jašková, V., Hochmannová, L., Vytrasová, J.,
2013. TiO2 and ZnO nanoparticles in photocatalytic and hygienic coatings.
International Journal of Photoenergy, Volume 2013. pp. 1–6
Karakas, F., Çelik, M.S., 2018. Stabilization
mechanism of main paint pigments. Progress in Organic Coatings. Elsevier
Volume 123, pp. 292–298
Li, Q., Liu, Y., Wan, Z., Cao, H., Zhang, S.,
Zhou, Y., Ye, X., Liu, X., Zhang, D., 2021. Microwave-assisted synthesis of
oxygen vacancy associated TiO2 for efficient photocatalytic nitrate
reduction. Chinese Chemical Letters, Volume 33(8), pp. 3835–3841
Lv, J., Yang, J., Li, X., Chai, Z., 2019. Size dependent radiation-stability
of ZnO and TiO2 particles. Dyes and Pigments, Volume 164, pp. 87–90
Mazabuel-Collazos, A., Gómez,
C.D., Rodríguez-Páez, J.E., 2019. ZnO-TiO2 nanocomposites synthesized by wet-chemical
route: study of their structural and optical properties. Materials Chemistry
and Physics, Volume 222, pp. 230–245
Ma, J., An, W., Xu, Q., Fan, Q., Wang, Y.,
2019. Antibacterial casein-based ZnO nanocomposite coatings with improved water
resistance crafted via double in situ route. Progress in Organic Coatings,
Volume 134, pp. 40–47
Mamaghani, A.H., Haghighat, F., Lee, C.-S.,
2019. hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic
degradation of air pollutants: preparation, characterization, properties, and
performance. Chemosphere, Volume 219, pp. 804–825
Miklecic, J., Blagojevic, S.L., Petri?, M.,
Jirous-Rajkovic, V., 2015. Influence of TiO2 and ZnO nanoparticles
on properties of waterborne polyacrylate coating exposed to outdoor conditions.
Progress in Organic Coatings, Volume 89, pp. 67–74
Papp, I.Z., Alegría, A., Kónya, Z., Kukovecz,
Á., 2022. Investigation into the effect of ZnO nanorod coating on the thermal-mechanical and dielectric
properties of ITO coated PET. Materials Research Bulletin, Volume 149, p. 111701
Peruchi, R., Bartosiak, A., Zuchinali, F.F.,
Bernardin, A.M., 2021. Development of a water-based acrylic paint with
resistance to efflorescence and test method to determine the appearance of stains.
Journal of Building Engineering, Volume 35, p. 102005
Razali, M.N. Bin, Alkaf, A.A., Zuhan,
M.K.N.B.M., 2022. formulation of water-based white colour paint from waste
titanium dioxide. Materials Today: Proceedings, Volume 48(6), pp.
1905–1909
Rosales, A., Ortiz-Frade, L., Medina-Ramirez,
I.E., Godínez, L.A., Esquivel, K., 2021. Self-cleaning of SiO2-TiO2 coating: effect of sonochemical
synthetic parameters on the morphological, mechanical, and photocatalytic
properties of the films. Ultrasonics Sonochemistry, Volume 73, p. 105483
Sisca, V., Deska, A., Syukri, S., Zilfa, Z.,
Jamarun, N., 2021. Synthesis and characterization of CaO limestone from lintau buo supported by TiO2
as a heterogeneous catalyst in the production of biodiesel. Indonesian
Journal of Chemistry, Volume 21(4), pp. 979–989
Sofyan, N., Ridhova, A., Wu, J., Yuwono,
A.H., 2018. Characteristics of nano rosette TiO2 hydrothermally
grown on a glass substrate at different reaction time and acid concentration. International
Journal of Technology, Volume 9(6), pp. 291–319
Sofyan, N., Ridhova, A., Yuwono, A.H.,
Sianturi, M.C., 2019. Effect of NaCl addition on nano rosette TiO2 crystal
growth during hydrothermal deposition. International Journal of Technology,
Volume 10(6), pp. 291–319
Solanki, K., Parmar, D., Savaliya, C., Kumar,
S., Jethva, S., 2021. Surface morphology and optical properties of sol-gel
synthesized TiO2 nanoparticles: Effect of Co, Pd and Ni-doping. Materials Today: Proceedings, Volume 50(part 6), pp.
2576–2580
Somturk, S.M., Emek, ?.Y., Senler, S., Eren,
M., Kurt, S.Z., Orbay, M., 2016. Effect of wollastonite extender on the
properties of exterior acrylic paints. Progress in organic coatings,.
Elsevier Volume 93, pp. 34–40
Song, S. Song, H., Li, L., Wang, S., Chu, W., Peng, K., Meng, X., Wang, Q., Deng, B., Liu, Q., Wang, Q., Weng, Y., Hu, H., Lin, H., Kako, T., Ye, J., 2021. A selective Au-ZnO/TiO2 hybrid
photocatalyst for oxidative coupling of methane to ethane with dioxygen. Nature
Catalysis, Volume 4(12), pp. 1032–1042
Yuwono, A.H., Zhang, Y., Wang, J., 2014. Investigating the nanostructural
evolution of TiO2 nanoparticles in the sol-gel derived TiO2-polymethyl methacrylate
nanocomposites. International Journal of Technology, Volume 1(1), pp.
291–319
