Microwave Assisted Hydrotropic Distillation of Myrcene-Rich Essential Oil of Cymbopogon Citratus
Corresponding email: hartatiprasetyo@gmail.com
Published at : 04 Apr 2023
Volume : IJtech
Vol 14, No 2 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i2.5381
Sediawan, W.B., Hartati, I., Sulistyo, H., Azis, M.M., Al Rahma, U., 2023. Microwave Assisted Hydrotropic Distillation of Myrcene-Rich Essential Oil of Cymbopogon Citratus. International Journal of Technology. Volume 14(2), pp. 310-319
| Wahyudi Budi Sediawan | Department of Chemical Engineering, Universitas Gadjah Mada, Jl. Grafika 2 Yogyakarta 55281, Indonesia |
| Indah Hartati | Department of Chemical Engineering, Universitas Wahid Hasyim, Jl. Menoreh Tengah X No 22 Semarang 55232, Indonesia |
| Hary Sulistyo | Department of Chemical Engineering, Universitas Gadjah Mada, Jl. Grafika 2 Yogyakarta 55281, Indonesia |
| Muhammad Mufti Azis | Department of Chemical Engineering, Universitas Gadjah Mada, Jl. Grafika 2 Yogyakarta 55281, Indonesia |
| Ulfia Al Rahma | Department of Chemical Engineering, Universitas Gadjah Mada, Jl. Grafika 2 Yogyakarta 55281, Indonesia |
Myrcene
can be used as a versatile starting material for a myriad product for various
applications. The drawbacks of the available myrcene separation techniques
promote the investigation of application of environmentally clean, faster, more
powerful, and cheaper methods. The combination of microwave heating system and
application of urea in hydrotropic-based distillation of myrcene-rich essential oil of Cymbopogon
Citratus was investigated in this work. A mathematical model of microwave assisted hydrotropic distillation (MAHtD)
of essential oil based on the diffusion-control assumption is also proposed and
validated. The MAHtD of C. citratus were performed by varying urea
concentrations (20-30%) and temperature (105-110°C). The essential oil
composition as well as its concentration were analyzed and determined by
applying GC-MS. Research result shows that urea is proved as a good hydrotrop
for MAHtD of myrcene–rich essential oil of C. citratus. MAHtD
performed with 25% urea at a temperature of 105°C for 10 minutes gives a high
myrcene content of up to 53.53%. The obtained myrcene–rich essential oil of C.
citratus can be investigated for its utilization in various types of
applications. Moreover, the proposed model was validated with the experimental
data of MAHtD performed at a solid-liquid ratio of 1:10, 25% urea solution, and
a temperature of 105°C. The experimental data show a good agreement with the
proposed model with SSE of 0.033. The obtained effective diffusivity of the
essential oil and the Henry constant in which essential for industrial scale-up
process are 2.245 and 0.42,, respectively. The effective diffusivity value was higher than those
obtained from hydro distillation and microwave-assisted hydro distillation of
other essential oil, which shows that urea, is a good solubilizing agent for C.
citratus essential oil.
Cymbopogon citratus; Hydrotropic; Microwave; Myrcene
Myrcene (C10H16), an acyclic monoterpene
in two isomeric forms ( myrcene), is derived from the Myrcia species
plant family. Bay leaves, verbena, pine, lemongrass, rosemary, juniper, rose,
ginger, and celery are some of myrcene plant sources (Bai and Tang 2020). Beta-myrcene is
often denoted as myrcene. The highly active diene structure of myrcene makes it
a versatile starting material for menthol, geraniol, nerol, and linalool
technical synthesis (Behr and Johnen 2009). Different types of
myrcene derivatives are applied in various applications, such as fragrances,
cosmetics, soaps, vitamins, pharmaceuticals, polymers, insect repellents,
flavors, and biodegradable surfactants (Behr and Johnen 2009). Some
literature also mention that myrcene exhibits biological activities such as
anti-diabetic, antioxidant, anti-cancer, analgesic, anti-inflammatory, anti-biotic,
sedative and anti-mutagenic properties (Bai and Tang 2020).
Industrial myrcene is
produced from the pyrolysis of pinene, catalytic dimerization of isoprene,
and the extraction from myriad myrcene plant sources (Behr and Johnen 2009). Hydro
distillation, solvent extraction, and supercritical fluid extraction of myrcene
are methods applied in the myrcene separation process. Hydro distillation and
solvent extraction are commonly performed at high temperatures and require
three-to-four hours of process. The possibility of losing the essential
volatile compound and altering its odor characteristics are two examples of
both techniques' drawbacks. Meanwhile, supercritical fluid extraction is
effective, but the device is highly sophisticated, uneconomical, and
operationally complex. The drawbacks of
myrcene separation techniques promote the demand for investigating the
potential application of environmentally clean, faster, more powerful, and
cheaper methods.
Hydrotropes are chemicals
that increase the solubility of hydrophobic molecules, such as monoterpene in a
water-based hydrotrope solution (Hartati et al. 2021). Hydrotropic
solubilization has been applied in the separation of terpene, such as
andrographolide from Andrographis paniculata Ness (Hartati,
Anas, and Kurniasari 2015), abiatane
diterpenoid (carnosic acid) from rosemary (Mazaud et al. 2020), and eugenol from
clove buds (Ghule and Desai 2021). Sodium benzoate is
applied in the hydrotropic separation of andrographolide (Hartati, Anas, and Kurniasari 2015). Short-chain alkyl
polyethyene glycol ethers are used to separate carnosic acid from rosemary (Mazaud et al. 2020). Sodium salicylate
and sodium cumene sulfonate are utilized in the extraction of eugenol and
eugenol acetate (Ghule and Desai 2021). Urea, a cheap and
environmentally friendly compound, is one of hydrotropes utilized in
solubilization of several poorly-water soluble compounds as nifedipine (Cui 2013) and rice straw
lignin (Hartati et al. 2021).
2.1. Materials
Cymbopogon citratus leaves were collected from Kalisidi, West Ungaran, Central Java. They were withered for 24 hours at room temperature and chopped into 1 cm length. Urea ((NH2)2CO) with a purity of 99% was produced by Pupuk Kujang Inc.
Figure 1 Microwave distillation unit
2.2. Procedure
Sixty g of dried and chopped C. citratus
leaves, and 600 mL of urea solution were placed in the three-neck glass jar and
inserted into the microwave distillation unit (EMM2308X model from Electrolux)
with a condenser and temperature control (Figure1). The microwave power level
was set to a high level (800 W), and the microwave-assisted hydrotropic
distillation (MAHtD) was
performed at a temperature of 110°C. The microwave distillation unit is
completed with the temperature control system (Figure 1). Samples were taken
and separated using a pipette, and the oil volume was recorded.
2.3.
GC-MS analysis
The essential oil was taken using a dropping pipette
to measure its chemical compound using a Shimadzu single quadrupole GCMS-QP2010
SE type of GC-MS. The GC- MS non-polar fused silica capillary column has an inside
diameter of 0.25 mm, a length of 30 m, and a film thickness of 0.25 mm. The
column temperature was initially programmed at 60°C and increased at 3°C/min to
150°C and 10°C/min to 250°C. The
final temperature was maintained for 15 minutes, while the injector and
detector temperature was set to 250°C. Helium gas was used as the carrier gas
with a flow rate of 0.52 mL/min, a total flow of 8.7 mL/min, a linear velocity
of 26.3 cm/sec, a purge flow of 3.0 mL/min, and a split ratio of 1:10.
where is
the essential oil concentration in the bulk of the liquid, and H is the Henry constant. The boundary condition
(Equation 4 and Equation 5) are obtained by assuming that mass transfer of
essential oil from solid surface to the bulk of the liquid is relatively fast
so that the essential oil concentration in the solid surface is equal to the one
in the liquid phase and thus is presented in an equation similar to Henry law.
3.1. Microwave assisted hydrotropic distillation of C. citratus
3.1.1. The effect of hydrotrope concentration
Figure 2 Essential oil yields of MAHtD of C. citratus performed with: (a)
20-30% of urea solution at a temperature of 110°C for 10-15 minutes, (b) 20-30%
of urea solution at a temperature of 105-110°C for 15 minutes
The
research showed that in two different process durations applied, yield of C.
citratus essential oil increase with the increase of urea concentration
used. Cymbopogon citratus essential oil yield of 0.5% was obtained from
MAHtD with 20% urea solution while 0.88% was obtained from MAHtD with 30% urea
solution. Other researchers
have reported similar results using hydrotropic-assisted distillation processes
for essential oils. The solubility of citral, eugenol, and eugenol acetate
increased with the increase of hydrotropic solution (NaSal and NaCuS) applied. The ability of hydrotropes
to solubilize solutes is attributed to the hydrotrope self-aggregation
characteristic. Some hydrotropes are able to aggregate in a step-wise
self-aggregation process and gradually increase aggregation size. It is also
mentioned that the other possible mechanism for hydrotropic solubilization
process is due to complexation (Choudhary and Nayal 2019).
Table 1 shows that yield of the MAHtD process applied
here was higher than those obtained from microwave assisted hydro distillation
(MAHD) of C. citratus. A yield of 0.67% was obtained from MAHD of 24
hours of withered leaves at a temperature of 105°C for 20 minutes. It showed
that urea solution is able to increase the solubility of essential oil and has
the ability to break the cell wall of leave where the essential oil is located.
Essential oil of was detected to localized, and stored at the different parts
of plants depending upon the producing species. Typically, essential oils are
stored in secretory cells, glandular trichomes, secretory ducts, and secretory
cavities (Saullea et al. 2018).
Table 1 Cymbopogon Citratus essential oil yield from
various processes
|
No. |
Process |
Operation Condition |
Yield (%) |
References |
|
1 |
MAHtD |
24 h of
withered leaves, 1 cm, T = 110°C, S/L = 1:10, t = 15 minutes, 30% urea |
0.88 |
This
research |
|
2 |
MAHtD |
24 h of
withered leaves, 1 cm, T = 110°C, S/L = 1:10, t = 10 minutes, 30% urea |
0.83 |
This research |
|
3 |
MAHtD |
48 h of
withered leaves, 1 cm, T = 110°C, S/L = 1:10, t = 15 minutes, 30% urea |
1.0 |
This
research |
|
4 |
MAHtD |
24 h of
withered leaves, 1 cm, T = 105°C, S/L = 1:10, t = 15 minutes, 30% urea |
1.03 |
This
research |
|
5 |
MAHD |
24
hours of withered leaves, 1 cm, T = 105°C, S/L = 1:10, t = 10 minutes |
0.33 |
This
research |
|
6 |
MAHD |
24
hours of withered leaves, 1 cm, T = 105°C, S/L = 1:10, t = 20 minutes |
0.67 |
This
research |
|
7 |
Distillation |
Dried
leaves, 3 cm, t = 2 hours, bed volume 80%, |
0.53 |
(Alam et al. 2018) |
|
8 |
MAHD |
Dried
leaves of 1 week drying process, grinded, 250W, S/L = 1:6, t = 90 minutes |
0.89 |
(Mathialagan, Nour, and Nour 2014) |
3.1.2. The effect of Temperature
The MAHtD were conducted at two different
temperatures, i.e. 105 and 110°C. Hydrotropic solubilization of solute is known
to increase with increasing temperature. The effect of temperature on the
aggregation behavior of aqueous solutions of sodium cumene sulfonate was
investigated (Wagle, Kothari, and Gaikar 2007). It is found that the enthalpy of micellization is lowered with
increasing temperature. Although the increasing temperature usually positively
affects solute solubilization, in this research, the yield of MAHtD performed
at a lower temperature (105°C) gives better yield than the one performed at 110°C
(Figure 2b). The decreasing yield at high temperature process could be caused
by the increase of thermal degradation of essential oil component. Essential
oil is comprised of various aromatics compounds such as terpenes. In high
temperature, terpenes could be converted into resinous products due to
oxidation and poly condensation reaction (Abylaeva et al. 2020).
Table 2 Chemical composition of Cymbopogon citratus essential oil
obtained from MAHtD performed 30% of urea solution, temperature of 105°C for 10
minutes
|
Peaks |
Retention time (min) |
Concentration (%) |
Compounds |
|
1 |
6.027 |
14.48 |
Beta.-Myrcene |
|
2 |
10.837 |
21.46 |
6-Methyl-5-hepten-2-one |
|
3 |
13.031 |
7.65 |
3,5-Heptadienal,
2-ethylidene-6-methyl- |
|
4 |
18.614 |
11.45 |
Linalool |
|
5 |
23.819 |
1.13 |
Z-Citral |
|
6 |
25.744 |
1.27 |
E-Citral |
|
7 |
26.699 |
12.63 |
Neryl Acetate |
|
8 |
26.971 |
1.20 |
Beta.-Citronellol |
|
9 |
29.920 |
28.73 |
Geraniol |
3.1.3. Chemical composition of C. citratus essential oil
The essential oil of Cymbopogon citratus obtained by
MAHtD performed with 25% and 30% of urea; the temperature of 105°C for 10
minutes was subjected to GC-MS analysis. The chromatogram of both samples
is depicted at Figures 3 and 4. It was found that the C.
citratus essential oil obtained from MAHtD performed with 30% of urea is
dominated by geraniol (28.73%), 6-Methyl-5- hepten-2-one (21.46%), and myrcene
(14.48%) (Table 2). A higher myrcene content was obtained from MAHtD performed
with 25% of urea solution, where the C. citratus essential oil obtained
is dominated by Beta-myrcene (53.53%), geraniol (14.23%) and 6-
Methyl-5-hepten-2-one (12.57) (Table 3).
Table 3 Chemical composition of Cymbopogon citratus essential oil
obtained from MAHtD performed 25% of urea solution, the temperature of 105°C
for 10 minutes
|
Peaks |
Retention
time (min) |
Concentration
(%) |
Compounds |
|
1 |
6.071 |
53.53 |
Beta-Myrcene |
|
2 |
7.751 |
0.77 |
cis-Ocimene |
|
3 |
10.846 |
12.57 |
6-Methyl-5-hepten-2-one |
|
4 |
13.033 |
0.82 |
Bicyclo[3.1.1]hept-3-en-2-one,
4,6,6-tri |
|
5 |
18.608 |
3.66 |
Linalool |
|
6 |
23.820 |
2.86 |
Z-Citral |
|
7 |
25.747 |
6.19 |
E-Citral |
|
8 |
26.689 |
3.02 |
Neryl
Acetate |
|
9 |
26.972 |
1.57 |
Beta.-Citronellol |
|
10 |
28.157 |
0.78 |
Nerol |
|
11 |
29.924 |
14.23 |
Geraniol |
Figure
4 The chromatography of Cymbopogon citratus
essential oil obtained from MAHtD performed 25% of urea solution, the temperature
of 105°C for 10 minutes
The
myrcene content of essential oil obtained from this research is relatively
high. The myrcene content was in the range of 3.18-7.68% for three different
maturity levels of the C. citratus plants in 6 hours of hydro
distillation process at a temperature of 100°C (Tajidin 2016). Meanwhile, a relatively high myrcene content of C. citratus
essential oil was mentioned by researcher investigating the effect of drying
methods towards the chemical composition of C. citratus essential oil
obtained from three hours of the hydro distillation process. Fresh, sun drying,
shaded, and oven drying of C. citratus leaves gave essential oil having
myrcene content up to 15.69, 16.16, 14.49 and 15.42%, respectively (Hanaa 2012). It was reported that the myrcene content of C. citratus is
27.83% (Gbenou et al. 2014) while hydro distillation of C.
citratus from Cuba was reported to give 6.52% of myrcene in its essential
oil (Pinto; et al. 2015). The high myrcene content of C. citratus obtained from this
research indicates that geraniol undergoes dehydration and isomerization during
MAHtD, resulting in the production of myrcene.
3.2. Mathematical model of microwave assisted
hydrotropic distillation of C. citratus
Figure 5 (a) Profile of the calculated and experimental data of
extraction percentage of MAHtD process performed with solid-liquid ratio of
1:10; 25% of urea and temperature of 105°C; (b) Profile of essential oil concentration as axial position and
time function
Validation of the mathematical model gives us a better
understanding of the mechanism of MAHtD as it could provide us with the profile
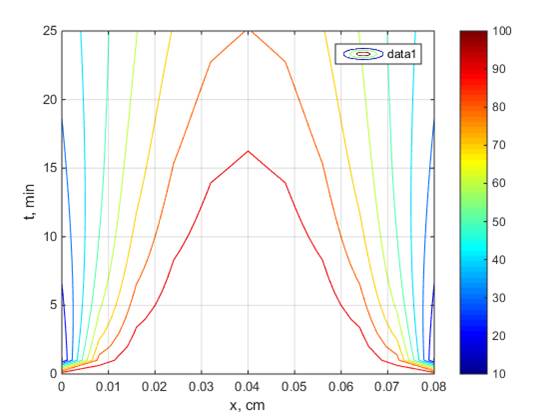
of essential oil concentration as axial position and time function (Figure 5b).
It shows that initially, the essential oil in the center of the slab is high.
It is logical since the hydrotrope solution is diffused from the outer part of the
solid particle towards the center of the solid particle. During the diffusion,
it solubilized the essential oil. As the separation process proceeded, the
essential oil concentration started to decrease as it diffused into the surface
of the solid particle's outer part.
Urea is proven as a good hydrotrope for
MAHtD of myrcene–rich essential oil of Cymbopogon citratus since MAHtD
of myrcene–rich essential oil of Cymbopogon citratus performed with 25% urea at a temperature of 105°C for 10 minutes resulted in a high myrcene content
of up to 53.53%. The proposed model was validated with the experimental data of
MAHtD performed at solid-liquid ratio of 1:10, 25% of urea solution, and a
temperature of 105°C. The experimental data show a good agreement with the
proposed model with an SSE of 0.033. The obtained effective diffusivity of the
essential oil is 2.245 and the Henry constant is 0.42. The effective diffusivity value was higher than
those obtained from hydro distillation and microwave-assisted hydrodistillation
of other essential oil, which shows that urea a good solubilizing agent for C.
citratus essential oil. The development and validation of mathematical
model of MAHtD gives us a better understanding of the mechanism of MAHtD and
also provide basic data that are essential for design of process equipment of
commercial scale. The scale up of the microwave assisted distillation unit in
larger scale is remaining as the main challenge of the application of
microwave-based processes. However, the availability and the relatively low
cost of urea as well as the high content of myrcene content in essential oil of
C. citratus obtained from MAHtD are the three main attractiveness of the
application of this method.
The authors greatly
acknowledge The Ministry of Research, Technology and Higher Education of
Republic of Indonesia, which supported this work through the PDUPT research
grant of 2021, with the contract number 1722/UN1/DITLIT/DIT-LIT/LT/2021 and
6564/UN1/DITLIT/DIT-LIT/LT/2021.
Abylaeva, B.,
Abdulazizov, T., Sandybaeva, Z., 2020. Essential oil from ether-containing
plants of juniper (juniperus) and spruce (picea) leaves by distillation. Green and Sustainable Chemistry, Volume
10(4), pp.109–11
Alam, P.N., Husin, H., Asnawi, T.M.,
Adisalamun., 2018. Extraction of citral oil from lemongrass (cymbopogon
citratus) by steam-water distillation technique. In: IOP Conference Series: Materials Science and Engineering,
Volume 345, p. 012022
Bai, X., Tang, J., 2020. Myrcene Exhibits antitumor
activity against lung cancer cells by inducing oxidative stress and apoptosis
mechanisms. Natural Product
Communications, Volume 15(9), pp. 1–7
Behr, A., Johnen, L., 2009. Myrcene as a natural
base chemical in sustainable chemistry: a critical review. Chemistry &
Sustainability Energy & Materials, Volume 2(12),
pp.1072–1095
Cassel, E., Vargas, R.M.F., 2006.
Experiments and modeling of the cymbopogon winterianus essential oil extraction
by steam distillation. Journal of the
Mexican Chemical Society, Volume 50(3), pp. 126–129
Choudhary, A.N., Nayal, S., 2019. A review: hydrotropy a solubility
enhancing technique. Pharma Innovation Journal,
Volume 8(4), pp. 1149–1153
Cui, Y., 2013. Hydrotropic solubilization
by urea derivatives: a molecular dynamics simulation study. Journal of Pharmaceutics, Volume 2013,
pp. 1–15
Ghule, S.N., Desai, M.A., 2021. Intensified
extraction of valuable compounds from clove buds using ultrasound assisted
hydrotropic extraction. Journal of
Applied Research onMedicinal and Aromatic Plants, Volume 25, p. 100325
Gbenou, J.D., Ahounou, J.F., Akakpo, H.B.,
Laleye, A., Yayi, E., Gbaguidi, F., 2013. Phytochemical composition of
cymbopogon citratus and eucalyptus citriodora essential oils and their
anti-inflammatory and analgesic properties on wistar rats. Molecular Biology Report, Volume 40(2), pp.1127–34
Hanaa, A.M., Sallam,
Y.I., El-Leithy, A.S., 2012. Lemongrass (cymbopogon citratus) essential oil
as affected by drying methods. Annals of
Agricultural Science, Volume 57(2), pp 113–116
Harahap, A.F.P., Rahman, A.A., Sadrina,
I.N., Gozan, M. 2019. Optimization of
pretreatment conditions for microwave-assisted alkaline delignification of empty
fruit bunch by response surface methodology. International Journal of
Technology, Volume 10 (8), pp. 1479–1487
Hartati, I., Anas, Y., Kurniasari, L., 2015.
Standardization of sambiloto (andrographis paniculata ness) extract obtained by
hydrotropic microwave assisted extraction. International Journal of Pharm
Tech Research, Volume 8(10)
Hartati, I., Sulistyo, H., Sediawan, W.B.,
Azis, M.M., Fahrurrozi, M., 2021. Microwave- assisted urea-based-hydrotropic
pretreatment of rice straw: experimental data and mechanistic kinetic models. ACS Omega, Volume 6(20), pp.13225–13239
Kusuma, H.S., Mahfud, M., 2015. Preliminary study: kinetics of oil
extraction from sandalwood by microwave-assisted hydrodistillation. Asean Journal of Chemical Engineering, Volume 15, pp. 62–69
Megawati, Fardhyanti, D.S., Sediawan, W.B., Hisyam, A., 2019. Kinetics
of mace (Myristicae arillus) essential oil extraction using microwave assisted hydrodistillation:
Effect of microwave power. Industrial
Crops and Products, Volume 131(2019), pp. 315–322
Ranitha, M., Abdurahman, N.H., Ziad, A.S., Azhari, N.H. and Thana
Raj, S., 2014. Comparison of essential oil of lemongrass (cymbopogon citratus)
extracted with Microwave-Assisted Hydrodistillation (MAHD) Conventional
Hydrodistillation (HD) method. Australian Journal of Basic and Applied
Sciences, Volume 8(19), pp.72–76
Maulida, A., Zahrati, Kamila, H., Mukhriza,
T., Gani, A., Supardan, M.D., 2020. Intensification
of synthesis of fatty acid isopropyl ester using microwave. International Journal of Technology,
Volume 11(3). P. 492–500
Mazaud, A., Lebeuf, R., Laguerre, M.,
Nardello-Rataj, V., 2020. Hydrotropic extraction of carnosic acid from rosemary
with short- chain alkyl polyethylene glycol ethers. ACS Sustainable Chemistry & Engineering, Volume 8(40),
pp.15268?15277
Paramita, V., Yulianto, M.E., Hartati,
I., 2019. Kinetic modelling and activation energy on microwave-integrated enzymatic-leaching
of vanillin from dried vanilla pods. Rasayan Journal of Chemistry, Volume
12(2), pp.484–493
Dao, T.P., Tran, N.Q., Tran, T.T., 2022. Materials today:
proceedings assessing the kinetic model on extraction of essential oil and
chemical composition from lemon peels (citrus aurantifolia) by
hydro-distillation process. Materials
Today Proceedings, Volume 51, pp
172–177
Saulle, C.C., Raman, V., Oliveira, A.V.G.,
Meneghetti, E.K., Flores, T.B., 2018. Anatomy and volatile oil chemistry of
eucalyptus saligna cultivated in South Brazil. Revista Brasileira de Farmacognosia, Volume 28, pp. 125–134
Tajidin, N.E., 2012. Chemical composition and
citral content in lemongrass (cymbopogon citratus) essential oil at three
maturity stages. African Journal of
Biotechnology, Volume 11(11), pp. 2685–2693
Wagle, I.B., Kothari,
W., Gaikar, V.G., 2007. Effect of temperature on aggregation behavior of
aqueous solutions of sodium cumene sulfonate. Journal of Molecular Liquids, Volume 133, pp. 68–76
Xiong, K., Chen Y., Shen, S., 2021.
Experimental optimization and mathematical modeling of supercritical carbon
dioxide extraction of essential oil from pogostemon cablin. Chinese Journal of Chemical Engineering,
Volume 27(10), pp. 2407–2417
Yustanti, E., Trenggono, A., Manaf, A.,
2020. Physical and microwave absorption
characteristics of high powered ultrasonically irradiated crystalline
BaFe9Mn1.5Ti1.5O19 particles. International
Journal of Technology, Volume 11 (2), pp. 310–321
