Hydroxylation Kinetics of Epoxidized Tung Oil Using Methanol as Nucleophilic Agent
Published at : 28 Jul 2023
Volume : IJtech
Vol 14, No 5 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i5.5376
Budiyati, E., Rochmadi, Budiman, A., Budhijanto, 2023. Hydroxylation Kinetics of Epoxidized Tung Oil Using Methanol as Nucleophilic Agent. International Journal of Technology. Volume 14(5), pp. 1060-1071
| Eni Budiyati | Department of Chemical Engineering, Faculty of Engineering, Universitas Muhammadiyah Surakarta, Jl. A. Yani No. 157, Pabelan Kartasura, Sukoharjo, Central Java, 57169, Indonesia |
| Rochmadi | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Sinduadi, Sleman, Yogyakarta, 55284, Indonesia |
| Arief Budiman | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Sinduadi, Sleman, Yogyakarta, 55284, Indonesia |
| Budhijanto | Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Sinduadi, Sleman, Yogyakarta, 55284, Indonesia |
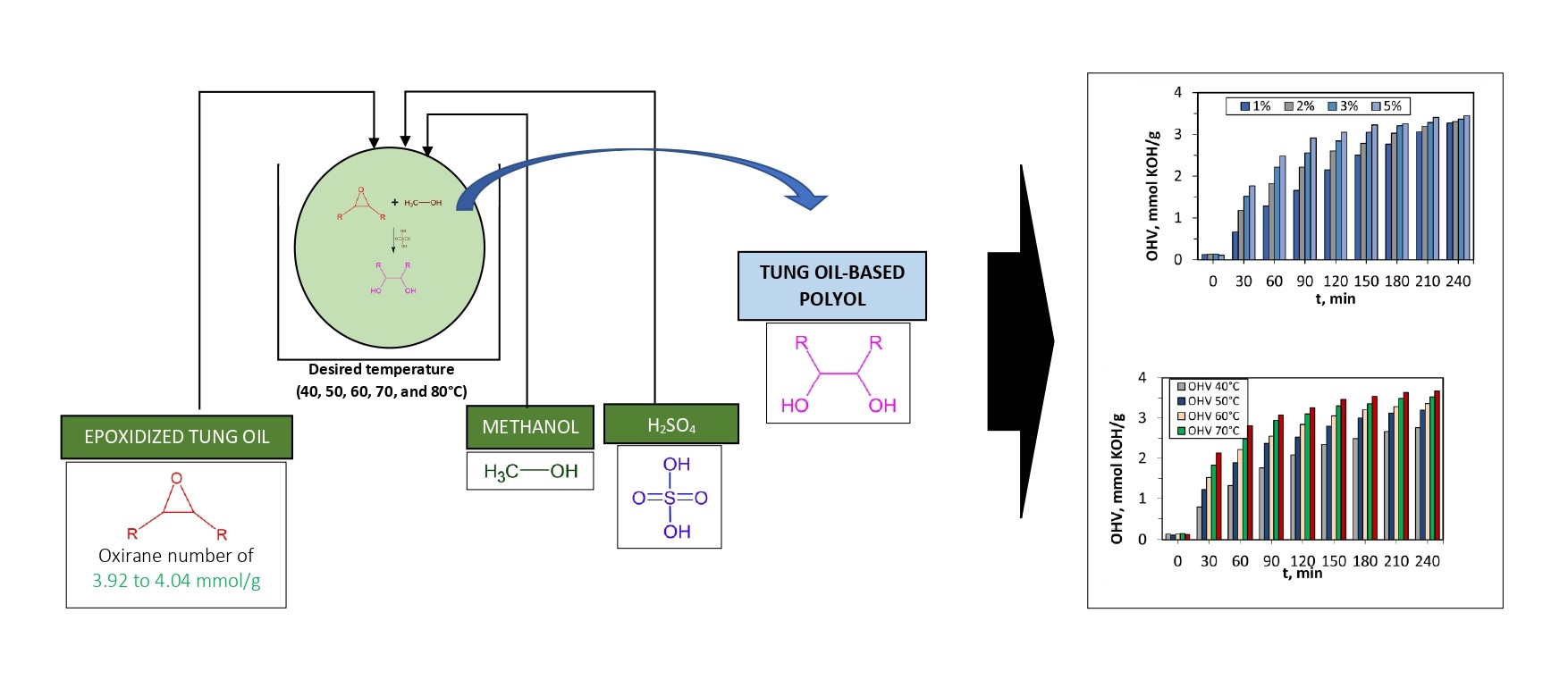
The synthesis of vegetable oil-based polyols has
been carried out through the epoxidation-hydroxylation process. However, Tung
oil has never been explored in the process, even though Tung oil has a high
double bond. Epoxidized
Tung Oil (ETO) with oxirane numbers of 3.92 to 4.04
mmol/g were hydroxylated with methanol and catalyzed by Sulfuric
Acid (H2SO4).
The objective of this study is to produce polyols from ETO and to evaluate the
influence of temperature and catalyst concentration on the hydroxylation process.
A second-order reaction kinetic study was also developed to represent the
reaction. The ETO hydroxylation was carried out in a batch reactor for four
hours. The reaction rate was determined based on the concentration of ETO
(oxirane number) and Hydroxyl Value (OHV). The result shows that temperature and
catalyst concentration have a directly proportional correlation to OHV and are
inversely proportional to the concentration of ETO (epoxy). For temperatures
above 40°C, the decreasing rate of the epoxy concentration at the beginning was
relatively much faster than at the end of the reaction. In the temperature
variation, the highest OHV obtained was 3.70 mmolKOH/g or 207.39 mgKOH/g at
80°C. The optimum catalyst concentration obtained in this study was 3%. The
evaluated kinetics model gave great results for all reaction temperatures, with
the highest total SSE for epoxy concentration and OHV data fitting of 0.156 and
0.045, respectively. The calculated activation energy (Ea) was 30.41 kJ/mol and
the pre-exponential factor (A) was 255.00 g.mmol-1.min-1.
Catalyst concentration; Epoxidized Tung Oil (ETO); Hydroxylation; Kinetics; Methanol
Polyol is an organic compound that has more than one hydroxyl (-OH) group. Bio-polyols that are synthesized from vegetable oils are a great alternative to replace petrochemical polyols. Oleochemical polyols have several characteristics, such as flexible, mechanical and chemical resistant, hard, and hydrophobic (Borowicz, Paciorek-Sadowska, and Isbrandt, 2020; Purnomo, Setyarini, and Anggono, 2020; Lavrenyuk, Parhomenko, and Mykhalichko, 2019). They are required in the manufacturing industries such as flooring, coatings, and foaming in the polymer industry, polyols are compounds that contain hydroxyl function groups that can be used for other chemical reactions. The main use of polyols is to be used as a reactant to make polymers. Polyols can also be used for other purposes, such as intermediate chemicals, lubricant formulations, and stabilizers (Bresolin et al., 2018; Gasni et al., 2017; Pavani, Pola-Rao, and Prasad, 2017; Kamalakar et al., 2015). Polyols are widely used in the manufacture of polyurethane. The synthesized polyol can be processed from renewable (e.g., vegetable oils) and unrenewable materials (e.g., petroleum). The hydroxyl group can be made from vegetable oils with a high content of unsaturated fatty acids through epoxidation followed by the hydroxylation process (Borowicz, Paciorek-Sadowska, and Isbrandt, 2020). Some vegetable oils have high unsaturated fatty acid content and some of them naturally contain other functional groups which cause various processes in synthesizing vegetable oil-based polyols. The four methods used to produce polyols are a) epoxidation followed by the ring-opening of the oxirane to form secondary hydroxyl groups; b) the enzymatic reaction; c) transesterification; and d) hydroformylation and reduction of aldehyde oil (Dong-Feng et al., 2019; Ionescu et al., 2016).
Hydroxylation
is the addition process of hydroxyl groups (-OH) to organic compounds.
Theoretically, vegetable oils can be converted to hydroxy in several ways, such
as catalytic hydrogenation, hydrochlorination or hydrobromination, and
ring-opening reactions with the addition of alcohol, amino alcohol, or acid (Coman et al., 2021; Borowicz,
Paciorek-Sadowska, and Isbrandt, 2020; Guo, Javni, and Petrovic, 2000). Hydroxylation reactions can be performed
in the absence of solvents with acidic catalysts. The catalysts used are
mineral acids, metal, and metal carbonyl. The ratio of molar hydroxyl and
epoxide groups ranges from 1:1 to 1:10 and the optimum temperature is around 50°C (Petrovic et al., 2010). In recent years, the ring-opening method of
epoxy has been widely investigated for the manufacturing process of vegetable
oil-based polyol compounds. This method involves two steps, i.e., epoxidation
of unsaturated fatty acids in vegetable oils and the ring-opening reaction of
epoxy using nucleophilic reagents such as amines, carboxylic acids/ halogenated
acids, or alcohol. The ring-opening reaction of epoxy with the addition of
alcohol produces alkoxy alcohol (Gallo, Teixeira, and Schuchardt, 2006; Williams, D Bradley, and Lawton, 2005). This reaction can be carried out using both
alkaline and acidic catalysts. Some of the catalysts that have been used in
these reactions include Lewis acid (Firouzabadi et al.,
2006), Bronsted acid (Gallo, Teixeira, and Schuchardt, 2006), and porphyrin complexes (Zakavi, Karimipour,
and Gharab, 2009).
Some researchers make polyols from
several vegetable oils, such as castor oil, flaxseed oil, sunflower seed oil,
soybean oil, peanut oil, and cotton seed oil through ozonolysis and
hydrogenation processes (Tran, Graiver, and Narayan, 2005) as well as epoxidation and
hydroxylation processes (Petrovic et al., 2010). Vegetable oils generally do not contain
hydroxyl groups but hold a relatively high content of unsaturated fatty acids (Helmiyati and Anggraini, 2019). The double bonding part of unsaturated
fatty acids can be converted into an epoxy group at atmospheric pressure
through epoxidation and further converted into a hydroxyl group with the
hydroxylation process. Petrovic et al.
(2010) patented epoxidized oil alcoholysis with different molecules such
as water, monoalcohol (methanol, ethanol, propanol, and butanol), and their
mixture using tetrafluoro boric acid as a catalyst (Petrovic et al., 2010; 2002). Polyol can be synthesized from several
epoxidized natural oils, such as epoxidized methyl oleate, epoxidized cocoa
butter, epoxidized palm olein, and epoxidized soybean. Polyols were produced
through a copolymerization reaction between the epoxidized oils and
tetrahydrofuran with a Lewis acid catalyst (Hoong et al., 2015). Mohammed et
al. (2013) synthesized polyols by reacting palm oil and soybean oil
with glycerol. The polyols were further processed in the manufacture of
polyurethane (Mohammed et al., 2013).
Tung oil is one of the types of
non-edible vegetable oil with a high content of unsaturated fatty acids. The
use of Tung oil as a raw material in the synthesis of polyols has several
advantages, such as: being renewable, relatively biodegradable, sustainable,
and not contradictory to food industry needs. Tung seeds kernel can result from
crude oil of 45-50% yield with a high content of unsaturated fatty acids, i.e.,
oleic acids, linoleic acids, and oleo stearate acids. The percentage of fatty
acid contained in Tung oil is about 66.17% of oleo stearic acid, 21.72% of
palmitic acid, 0.27 % of linoleic acid, 9.58% of oleic acid, and 2.26% of other
saturated fatty acids (Budiyati et al., 2020). Synthesis of bio-polyols can be carried out
by epoxidation of double bonds in unsaturated fatty acids and ring-opening of
epoxides with nucleophilic reagents. The advantages of chemical flow, such as
increased process safety and reliability, easy automation, and reproducibility
are leveraged to increase productivity and product quality in the ring-opening
epoxy. Pantone et al. (2017) investigated
the methanolysis reaction of Soy Oil Oxidized (ESO) in a continuous
flow system (Pantone et al., 2017). Various chemical modifications to Epoxidized Soybean Oil (ESBO) are performed through
the opening of epoxy rings. The ring-opening reaction with nucleophilic
compounds is one of the most commonly used processes. Several previous studies
examined the hydroxylation of epoxy rings with acids (acetic acid, formic acid,
and phosphoric acid) (Campanella and Baltanas, 2007; Guo et al., 2007). Alcoholysis using methanol, 1-decanol,
1-butanol, and 2-ethylhexanol was studied for the manufacture of lubricant
formulation (Hwang, Adhvaryu, and Erhan, 2003; Hwang and Erhan, 2001). The alkoxylate synthesis of triacylglycerol
from ESBO was developed using perchloric acid for the opening of epoxy rings (Adhvaryu, Liu, and Erhan, 2005).
Based on the literature review, the
hydroxylation process with Tung oil as raw material has never been carried out.
Evaluations of the hydroxylation kinetics and the process variables' effects
(such as temperature and catalyst concentration) also have not been discussed
specifically in the previous studies. Several previous studies evaluated the
reaction rate constants of the hydroxylation (epoxy and alcohol) as a part of
the side reaction in the epoxidation of vegetable oils (Kousaalya et al., 2018; De Haro et al.,
2016; Dai et al., 2009). Overall, this research has novelties in the
type of vegetable oil (i.e., Tung oil) and the proposed reaction kinetics.
Therefore, this study aims to evaluate the influence of temperature and
concentration of catalyst, as well as the second-order reaction kinetics in the
hydroxylation of ETO.
2.1. Materials
Epoxidized
Tung Oil (ETO) was
produced from the epoxidation process of Tung seed oil (Reutealis trisperma
(Blanco) Airy Shaw) (Budiyati et al., 2020). It has an initial concentration of epoxy
and hydroxy of 3.92 to 4.04 mmol/g and 0.11 to 0.16 mmol/g, respectively.
Pyridine, crystal violet indicator, hydrobromic acid (47 wt%), methanol,
sulfuric acid 98 wt%, glacial acetic acid (AR Grade), and potassium hydroxide
were purchased from CV. Zirconia Jaya, Yogyakarta, Indonesia. Acetic anhydride
and potassium hydrogen phthalate were purchased from CV. Multikimia,
Yogyakarta, Indonesia.
2.2.
Hydroxylation Process
Epoxidized Tung Oil (ETO) was produced from Tung seed oil
epoxidation. Peracetic acid was used as an oxidator (in-situ generated) and
sulfuric acid as the catalyst. The epoxidation process was carried out for 4
hours at a temperature of 60°C and the stirring speed was maintained constant
at 600 rpm (Budiyati
et al., 2021). ETO was
hydroxylated with 6 g of methanol and 3% (of the total solution) sulfuric acid
in an Erlenmeyer flask. As much as 75 g of epoxidized Tung oil and a mixture of
methanol and sulfuric acid were fed into the reactor. The process was carried
out isothermally (at a temperature of 40°, 50°, 60°, 70°, and 80°C) with a stirring
velocity of 700 rpm. Then, the catalyst concentration was varied at 1%, 2%, 3%,
and 5%. The Hydroxylation process ran for 4 hours with a sampling time interval
of 30 minutes. The samples were purified in the separation funnel using warm
distilled water. The obtained samples were analyzed to determine their oxirane
number and hydroxyl value. A schematic illustration of this experiment is shown
in Figure 1.
2.3
Determination of Concentration of ETO and Hydroxyl Value
The concentration of ETO was represented
by the oxirane number. It was analyzed by hydrobromic acid solution with
glacial acetic acid method (Budiyati et al., 2020). The Hydroxylation
Value
(OHV) of polyols was determined according to the ASTM method D1957-86 with the
mixture of pyridine and acetic anhydride as a hydroxyl number reagent.
Potassium hydroxide was used as a titrant in the titration process.
Figure 1 Schematic illustration of this experiment
3.1. Effect of Temperature on
Hydroxylation
Temperature significantly affects chemical
reactions, including ETO hydroxylation. In this study, the optimum temperature
evaluation was conducted. ETO as a reactant or raw material is naturally highly
reactive. Before the raw materials were used in experiments, they were stored
in the freezer with limited storage time (maximum about 2 days) at a
temperature of 4°C. After the freezing pre-treatment, the raw materials were
ready to be fed into the reactor which has varied temperatures of 40°, 50°,
60°, 70°, and 80°C. Figure 2(a) presents a correlation between epoxy/ETO
concentration and reaction time at various temperatures of hydroxylation. From
Figure 2(a), it is seen that the concentration of ETO decreased with the
increase in reaction time and this condition occurred at all observed reaction
temperatures. It is obvious because the longer the reaction time, the more ETO
moles (as reactants) reacted. As a consequence, there are more epoxy molecules
converted in the process. The temperature has a similar effect as the reaction
time to the hydroxylation of ETO. The rising temperature decreases the ETO
concentration. This phenomenon happens because the increase in temperature
enlarged the kinetic energy and reactiveness of the reactant molecules. The
decreasing rate of epoxy concentration at the lowest temperature, 40°C, was
relatively slow from the beginning to the end of the reaction. At higher
temperatures, 50° to 80°C, the decreasing rate of the epoxy concentration at
the beginning was relatively fast and began to stable after 120 minutes. As
mentioned previously, at a higher temperature the molecular kinetics of the
reactants will increase, thus increasing the collision factor and accelerating
the reaction rate (Yang et al.,
2010).
Methanol was a primary alcohol that can
be a powerful nucleophile in the hydroxylation of ETO. It is seen from the
final conversions (of about 84%) with a reaction time of 4 hours. This result
supports previous research which states that the hydroxylation conversion of
epoxide compounds with primary alcohol was higher compared to secondary and
tertiary alcohols (Mirza-Aghayan et al., 2014). Mirza-Aghayan,
et al. (2014) stated that a long reaction time is required for
the opening of epoxide rings with secondary alcohol. However, the generated
conversions in this study were lower than in previous studies. Hydroxylation of
styrene epoxide with methanol-catalyzed Graphite Oxide (GO)
resulted in a conversion of about 94%. This significant difference was due to
the use of different raw materials and catalysts (Mirza-Aghayan et
al., 2014).
Figure 2(b) shows OHV at various
reaction times and temperatures. The observed concentration of polyols or OHV
increased with reaction time, for all temperatures. This phenomenon occurs
because a longer reaction time increased particle contact. At the same reaction
time, the samples treated with higher temperatures have more OHV produced. The
molecular kinetics of the reactant molecules rose with the increase in
temperature and this condition triggered the collision molecules. The highest
OHV obtained in this study (from the optimum temperature variation) was
approximately 3.70 mmol KOH/g or 207.39 mg KOH/g. This result was in accordance
with the previous studies, which are 180.34 mg KOH/g (Dai et al., 2009) and 191±15 mg KOH/g (Pantone et al.,
2017). Dai et al. (2009) also
conducted similar research with different types of alcohol, namely
1,2-ethanediol, and 1,2-propanediol. The obtained OHV values are 253.33 mg
KOH/g (for 1,2-ethanediol) and 289.31 mg KOH/g (1,2-propanediol). The vegetable
oil used in the two aforementioned studies was soybean oil, while this research
also used vegetable oil. Based on these results, it can be concluded that the
type of vegetable oil and operating conditions are parameters that have a
significant effect on the results of the hydroxylation reaction.
3.2. Effect of The Catalyst
Concentration on Hydroxylation
The
evaluation of the catalyst's influence on ETO hydroxylation was conducted at
the temperature of 60°C and a constant stirring velocity of 600 rpm. The
catalyst used in this study was sulfuric acid (H2SO4). The catalyst activated methanol
as a nucleophilic agent, then activated methanol reacted with ETO. Catalyst
concentration is an important parameter in reaction kinetics. Catalysts can
accelerate reactions, but on the other hand, can produce burnt or dark brown
polyols. In this study, the catalyst concentrations were varied at 1%, 2%, 3%,
and 5% to obtain the optimum value.
Figure
3(a) describes the correlation between the concentration of epoxy/ETO against
the time and catalyst concentration. As explained in the previous discussion, a
longer reaction time resulted in a lower concentration of epoxy. Generally, the
concentration of catalysts had an inverse correlation to the ETO concentration.
Higher catalyst concentration promotes more epoxy molecules to react. Catalysts
reduce the activation energy; therefore, reactions can run faster. However, the
addition of a catalyst has its limit. The addition of catalyst concentrations
from 1% to 3% had a significant effect on the reaction kinetics while the
addition of catalysts from 3% to 5% produced a less significant effect.
The initial
concentration of ETO was around 3.99 – 4.00 mmol/g. The initial concentration
of ETO was not fixed at a single value due to the highly reactive properties of
epoxy compounds. In practice, ETO was prepared in a time adjacent to its
hydroxylation process. At the end of the reaction, the ETO converted were
approximately 75.58%, 77.46%, 80.34%, and 81.00% for catalyst concentrations of
1%, 2%, 3%, and 5%, respectively. In addition, the use of an H2SO4
catalyst at relatively high concentrations caused the discoloration of
polyols, i.e., dark brown. This can lead to denaturation of the product,
resulting in decreased quality of polyols. From these data, a catalyst
concentration of 3% can be recommended as the optimum value in this reaction.
These results differ slightly from those reported by Yadav
and Singh (2014), optimum catalyst concentration was 5% (Yadav and Singh, 2014). This was due to the different
types of catalysts and epoxy used. The types of catalysts used in the previous
study and this research are ferrocenium tetrafluoroborate and sulfuric acid,
respectively. Then, the previous study used non-vegetable oil-based epoxies,
such as 1,2-epoxydodecane and 1,2-epoxyhexane.
3.3. Hydroxylation Kinetics
The second-order reaction
model was evaluated on the hydroxylation kinetics. Two assumptions were taken
in this research: (1) the hydroxylation was a pseudo-homogeneous reaction and
(2) there is no side reaction occurred. The reason for taking the assumption
(1) is that methanol and ETO are organic compounds that can dissolve well with
each other. Then, sulfuric acid is an inorganic compound that has limited
solubility in these organic compounds. The amount of sulfuric acid is
inconsiderable (i.e., 3%). So, overall, the reaction can be considered a
pseudo-homogeneous. While, the reason for assumption (2) is that based on the
experimental results, the concentration of the oxirane (which reacts) is
proportional to the concentration of the formed polyol. The molar ratio of
epoxy (ETO) to methanol was 1:1 (equimolar). The concentration of the H2SO4
catalyst was 3% of the total weight of the reactants. The used catalyst
concentration was based on the discussion in the previous section (i.e.,
section 3.2.), which concluded that the optimum catalyst concentration was 3%.
The process was carried out in a batch reactor for four hours. The reaction
took place isothermally and at a constant stirring speed (700 rpm). The
mechanism of the ETO hydroxylation is shown in Equation (1).
The elementary reaction law states that the rate of a reaction is
proportional to the concentration of each reactant.
Then, the rate of reaction can be represented
by Equation (2).
Because of equimolar ([R3OH]0 = [ETO]0)
and coefficient of R3OH = coefficient of epoxy (ETO), so [R3OH]
= [ETO]. Then Equation (2) changed into Equation (3).
The reaction rate equation followed Equation (3), where the
hydroxylation had a reaction order of 2 or could be called a second-order
reaction.
The sum of the square of errors (SSE) of this
kinetics model was determined by Equation (4).
The experimental data used in this model were the ETO concentration and OHV for each temperature. The kinetic parameter (k) was obtained by minimizing the sum of the square of error (SSE) as the objective function. The algorithm for determining the reaction rate constant is shown in Figure 4.
Figure 4 Algorithm for determining the
reaction rate constant (k)
The experimental data
and resulting model concentrations of ETO and OHV are shown in Figure 5. From
Figure 5, it is clear that at all evaluated reaction temperatures, experimental
data on epoxy and polyol concentrations were fit to the modeling results. This
indicates that in the reaction system, there was no side reaction and the
reaction rate has an order of 2 (two). At 40°C, the deviation between the
experimental data and the model (especially for epoxy concentrations) was
relatively greater than at higher temperatures. For example, at a reaction time
of 30 minutes, the ETO concentrations were about 3.52 mmol/g (experiment) and
3.18 mmol/g (model). This was due to the high viscosity property of epoxidized
Tung oil. At the beginning of the reaction, this high viscosity causes poor
homogeneity in the reaction system and a low reaction rate. This high viscosity
was not considered in the model, as there is no viscosity parameter from
Equation (2) to (3). The deviation between the ETO concentration of
experimental data and the model was getting smaller by the time t > 30
minutes because homogeneity was achieved.
The
reaction rate constant and the Sum of the Square of Errors (SSE) for each temperature
are shown in Table 1. The reaction rate constant had a reaction order of 10-3
g.mmol-1.min-1. This result is in agreement with the
previous study (De Haro et al., 2016). The reaction rate constant was
directly proportional to the reaction temperature. The k values for the range
temperatures of 40 – 80°C were 1.98 x10-3 to 7.83 x10-3
g.mmol-1.min-1. The smallest SSE for epoxy and polyol
concentrations were obtained at 60°C and 70°C. In addition, the sample treated
at 40°C resulted in the highest SSE, with an average SSE of 0.100. From Table
1, it is seen that in the temperature range of 40°C to 70°C, the average SSE
value decreased with the increase in temperature. However, at a temperature of
80°C the SSE average rose again. It indicates that it was possible that the
side reactions began to occur at such a temperature. The observed SSE for
polyol concentrations/OHV was around 0.004 to 0.045 while the SSE of epoxy was
0.013 to 0.156. This suggests that experimental data from epoxy and polyol
concentrations were fit to the results of the model. Overall, the evaluated
kinetics model provided satisfactory results.
Figure 5 The experimental and model concentrations of
ETO and polyol (OHV) as a function of reaction time at various temperatures:
(a) 40°C, (b) 50°C, (c) 60°C, (d) 70°C, and (d) 80°C
Table 1 Reaction rate
constant (k) and SSE values for hydroxylation kinetics model
The reaction rate constants obtained from each temperature can be
further used as the input data to determine the Activation Energy (Ea)
and Pre-exponential
Factor (A) using the Arrhenius
equation. The relative error value of the curve was low and the r-square
parameter shows a satisfactory value of 0.98. The curve slope is the value of
(-Ea/R), where R is the ideal gas constant. Meanwhile, the intercept curve is
the ln (A) value. The calculated energy (Ea) is 30.41 kJ/mol and the Pre-exponential Factor (A) is 255.00 g.mmol-1.min-1.
The Ea values in this study were a bit different from the previous studies,
which were 29.56 and 39.99 kJ/mol (Dai et al., 2009) and 43.11 kJ/mol (Kousaalya et al., 2018). The vegetable oils used in the
previous study were soybean oil and Perilla oil, respectively. Overall, the
type of vegetable oil directly affects the value of the resulting Ea of
hydroxylation.
Polyols were successfully synthesized from epoxidized Tung oil
through hydroxylation using methanol as a nucleophilic agent. Sulfuric acid (H2SO4)
was added as a catalyst. From this study, it can be concluded that the
resulting polyol concentrations (OHV) were directly proportional to the
temperature, time of reaction, and catalyst concentration. On the other hand,
the concentration of ETO is inversely proportional to the mentioned parameters.
The highest OHV obtained from the temperature variation was 207.39 mg KOH/g at
80°C. A catalyst concentration of 3% can be recommended as the optimum
condition in this reaction. A proposed second-order reaction kinetics model can
represent hydroxylation kinetics. A good agreement between experimental data
and calculated concentration was achieved. The k values for the range
temperatures of 40°C – 80°C were about 1.98 x10-3 to 7.83 x10-3
g.mmol-1.min-1. Future research shall focus on the
characterization and application of Tung oil-based polyol products.
The authors are thankful to the
“Direktorat Riset dan Pengabdian Masyarakat, Direktorat Jenderal Penguatan
Riset dan Pengembangan, Kementerian Riset, Teknologi dan Pendidikan Tinggi”
Indonesia for the research grant of Penelitian Disertasi Doktor (PDD). Number
3079/UN1.DITLIT/DIT-LIT/PT/2020.
| Filename | Description |
|---|---|
| R1-CE-5376-20220306013407.docx | Graphical Abstract in DOC |
Adhvaryu,
A., Liu, Z., Erhan, S.Z., 2005. Synthesis of Novel
Alkoxylated Triacylglycerols
and Their Lubricant
Base Oil
Properties. Industrial Crops and Products, Volume 21(1), pp. 113–119
Borowicz,
M., Paciorek-Sadowska, J., Isbrandt, M., 2020. Synthesis and Application of New
Bio-polyols Based
on Mustard Oil
for the Production of Selected
Polyurethane Materials.
Industrial Crops and Products, Volume 155,
p. 112831
Bresolin,
D., Mazurek, V., Valério, A., Sayer, C., de Araújo, P.H.H., de Oliveira, D.,
2018. Poly (Urea-urethane)
Nanoparticles using Mono- and Diacylglycerol
from Glycerolysis of Castor
Oil as Biopolyol
and Stabilizer. European
Polymer Journal, Volume 108, pp. 529–535
Budiyati,
E., Rochmadi, Budiman, A., Budhijanto, 2021. Synthesis of Vegetable Oil-Based
Polymers from Tung Oil (Reutealis Trisperma(Blanco) AiryShaw). Universitas
Gadjah Mada
Budiyati,
E., Rochmadi, R., Budiman, A., Budhijanto, B., 2020. Studies on Epoxidation of Tung
Oil with Hydrogen
Peroxide Catalyzed
by Sulfuric Acid.
Bulletin of Chemical Reaction Engineering and Catalysis, Volume 15(3), pp. 674–686
Campanella,
A., Baltanás, M.A., 2007. Degradation of the Oxirane
Ring of Epoxidized
Vegetable Oils
in a Liquid-liquid-solid Heterogeneous Reaction
System. Chemical
Engineering and Processing, Volume
46(3), pp. 210–221
Coman,
A.E., Peyrton, J., Hubca, G., Sarbu, A., Gabor, A.R., Nicolae, C.A., Iordache,
T.V., Averous, L., 2021. Synthesis and Characterization
of Renewable Polyurethane
Foams using Different
Biobased Polyols
from Olive Oil.
European Polymer Journal, Volume 149, p.
110363
Dai,
H., Yang, L., Lin, B., Wang, C., Shi, G., 2009. Synthesis and Characterization of the Different Soy-based
Polyols by Ring
Opening of Epoxidized
Soybean Oil
with Methanol, 1,2-ethanediol and 1,2-propanediol. Journal
of the American Oil Chemists' Society, Volume 86, pp. 261–267
De-Haro,
J.C., Izarra, I., Rodríguez, J.F., Perez, A., Carmona, M., 2016. Modelling the Epoxidation Reaction
of Grape Seed
Oil by Peracetic
Acid. Journal of Cleaner Production, Volume 138, pp. 70–76
Dong-Feng, G., Ma, Y., Zhang, M., You-Jia, P., Hong-Hu, Li., Guo-Liu, C., Hong-Zhou, Y., 2019. Polyurethane-coated Urea using Fully
Vegetable Oil-based
Polyols: Design, Nutrient
Release and Degradation.
Progress in Organic Coatings, Volume 133,
pp. 267–275
Firouzabadi,
H., Iranpoor, N., Jafari, A.A., Makarem, S., 2006.
Aluminumdodecatungstophosphate (AlPW12O40) as a Reusable
Lewis Acid Catalyst: Facile Regioselective
Ring Opening
of Epoxides with Alcohols,
Acetic Acid
and Thiols. Journal of Molecular Catalysis A:
Chemical, Volume 250(1-2),
pp. 237–242
Gallo,
J.M.R., Teixeira, S., Schuchardt, U., 2006. Synthesis and Characterization of Niobium Modified
Montmorillonite and its use in the Acid-catalyzed Synthesis
of hydroxyethers. Applied Catalysis A: General, Volume 311, pp. 199–203
Gasni,
D., Mulyadi, I.H., Affi, J., Miswar, A.Y., 2017. Investigation of Wear
Mechanism in Ball Bearings Lubricated by A Bio-lubricant.
International Journal of Technology, Volume 7,
pp. 1248–1257.
Guo,
A., Javni, I., Petrovic, Z., 2000. Rigid Polyurethane
Foams Based
on Soybean Oil.
Journal of Applied Polymer Science, Volume 77(2), pp. 467–473
Guo,
Y., Hardesty, J.H., Mannari, V.M., Massingill, J.L., 2007. Hydrolysis of Epoxidized Soybean
Oil in the Presence
of Phosphoric Acid.
Journal of the American Oil Chemists' Society, Volume 84, pp. 929–935
Helmiyati, Y.A., Anggraini, Y., 2019. Nanocomposites Comprising Cellulose
and Nanomagnetite as Heterogeneous
Catalysts for the Synthesis
of Biodiesel from Oleic
Acid. International Journal of Technology, Volume 10(4), pp. 798–807
Hoong,
S.S., Yeong, S.K., Hassan, H.A., Din, A.K., Choo, Y.M., 2015. Synthesis and Characterization of Polyurethanes Made
from Copolymers of Epoxidized
Natural Oil
and Tetrahydrofuran. Journal of Oleo Science,
Volume 64(1),
pp. 101–115
Hwang,
H.S., Adhvaryu, A., Erhan, S.Z., 2003. Preparation and Properties of Lubricant
Basestocks from Epoxidized
Soybean Oil
and 2-ethylhexanol. Journal of the American Oil Chemists' Society, Volume 80(8),
pp. 811–815
Hwang,
H.S., Erhan, S.Z., 2001. Modification of Epoxidized
Soybean Oil
for Lubricant Formulations
with Improved Oxidative
Stability and Low
Pour Point.
Journal of the American Oil Chemists' Society, Volume 78, pp. 1179–1184
Ionescu,
M., Radojcic, D., Wan, X., Shrestha, M.L., Petrovic, Z.S., Upshaw, T.A., 2016.
Highly functional polyols from castor oil for rigid polyurethanes. European
Polymer Journal, Volume 84, pp. 736–749.
Kamalakar,
K., Mahesh, G., Prasad, R.B.N., Karuna, M.S.L., 2015. A Novel Methodology
for the Synthesis of Acyloxy
Castor Polyol
Esters: Low Pour
Point Lubricant
Base Stocks.
Journal of oleo science, Volume 64(12), pp. 1283–1295
Kousaalya,
A.B., Beyene, S.D., Gopal, V., Ayalew, B., Pilla, S., 2018. Green Epoxy Synthesized
from Perilla Frutescens: A Study
on Epoxidation and Oxirane
Cleavage Kinetics
of High-linolenic Oil.
Industrial Crops and Products, Volume 123,
pp. 25–34
Lavrenyuk,
H., Parhomenko, V.P., Mykhalichko, B., 2019. The Effect of Preparation
Technology and the Complexing on the Service
Properties of Self-extinguishing
Copper(II) Coordinated
Epoxy-amine Composites
for Pouring Polymer
Floors. International Journal of Technology, Volume 10(2),
pp. 290–299
Mirza-Aghayan,
M., Alizadeh, M., Tavana,
M.M., Boukherroub, R., 2014. Graphite Oxide: A Simple
and Efficient Solid
Acid Catalyst
for the Ring-opening of Epoxides
by Alcohols. Tetrahedron Letters, Volume 55(49),
pp. 6694–6697
Mohammed,
I.A., Al-Mulla, E.A.J., Kadar, N.K.A., Ibrahim, M., 2013. Structure-property Studies of Thermoplastic
and Thermosetting Polyurethanes
using Palm and Soya
Oils-based Polyols.
Journal of Oleo Science, Volume 62(12), pp. 1059–1072
Pantone,
V., Laurenza, A.G., Annese, C., Fracassi, F., Fusco, C., Nacci, A., Russo, A.,
D’Accolti, L., 2017. Methanolysis of Epoxidized
Soybean Oil
in Continuous Flow
Conditions. Industrial Crops and Products, Volume 109, pp. 1–7
Pavani,
P.N.L., Pola-Rao, R., Prasad, C.L.V.R.S.V., 2017. Synthesis and Experimental Investigation
of Tribological Performance
of a Blended (Palm
and Mahua) Bio-lubricant
using the Taguchi Design
of Experiment (DOE).
International Journal of Technology, Volume 8(3), pp. 418–427
Petrovic,
Z., Javni, I., Guo, A., Zhang, W., 2002. Method of Making Natural
Oil-based Polyols
and Polyuretahanes Therefrom.
United States Patent,
US 6,433,121 B1
Petrovic,
Z.S., Javni, I., Zlatanic, A., Guo, A., 2010. Modified Vegetable Oil-based
Polyol. United States Patent, US 7,786,239 B2
Purnomo,
P., Setyarini, P.H., Anggono, A.D., 2020. Fiber Orientation Effect on Fracture
Toughness of Silk Fiber-Reinforced Zeolite/HDPE Composites. Faculty of
Mechanical Engineering (FME) Transactions, Volume 49(1),
pp. 128–134
Tran,
P., Graiver, D., Narayan, R., 2005. Ozone-mediated polyol synthesis from
soybean oil. Journal of the American Oil Chemists Society, Volume 82(9),
pp. 653–659
Williams, D Bradley G., Lawton, M., 2005. Aluminium Triflate: A Remarkable
Lewis Acid Catalyst
for the Ring Opening
of Epoxides by Alcohols. Organic and Biomolecular Chemistry, Volume
3(18), pp. 3269–3272
Yadav,
G.D., Singh, S., 2014. Ring Opening of Epoxides With Alcohols Using Fe(Cp)2BF 4
as Catalyst. Tetrahedron Letters, Volume 55(29), pp. 3979–3983
Yang,
Z., Peng, H., Wang, W., Liu, T., 2010. Crystallization behavior of
poly caprolactone)/layered double hydroxide nanocomposites. Journal of
Applied Polymer Science, Volume
116(5), pp. 2658–2667
Zakavi,
S., Karimipour, G.R., Gharab, N.G., 2009. Meso-tetraarylporphyrin Catalyzed Highly
Regioselective Ring
Opening of Epoxides
with Acetic Acid.
Catalysis Communications, Volume 10(4), pp. 388–390
