A Facile Conjugation of 6-Hydroxylflavone Biomolecule with Polyethylene Glycol for Enhancing Conjugate Stability
Published at : 01 Jul 2022
Volume : IJtech
Vol 13, No 3 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i3.5336
Jamaludin, N.A., Harun, N.F.C., 2022. A Facile Conjugation of 6-Hydroxylflavone Biomolecule with Polyethylene Glycol for Enhancing Conjugate Stability. International Journal of Technology. Volume 13(3), pp. 575-583
| Nurul Aina Jamaludin | Universiti Kuala Lumpur- Branch Campus Malaysian Institute of Chemical and Bioengineering Technology, Lot1988, Kawasan Perindustrian Bandar Vendor, Taboh Naning, 78000, Alor Gajah, Melaka, Malaysia |
| Noor Faizah Che Harun | Universiti Kuala Lumpur- Branch Campus Malaysian Institute of Chemical and Bioengineering Technology, Lot1988, Kawasan Perindustrian Bandar Vendor, Taboh Naning, 78000, Alor Gajah, Melaka, Malaysia |
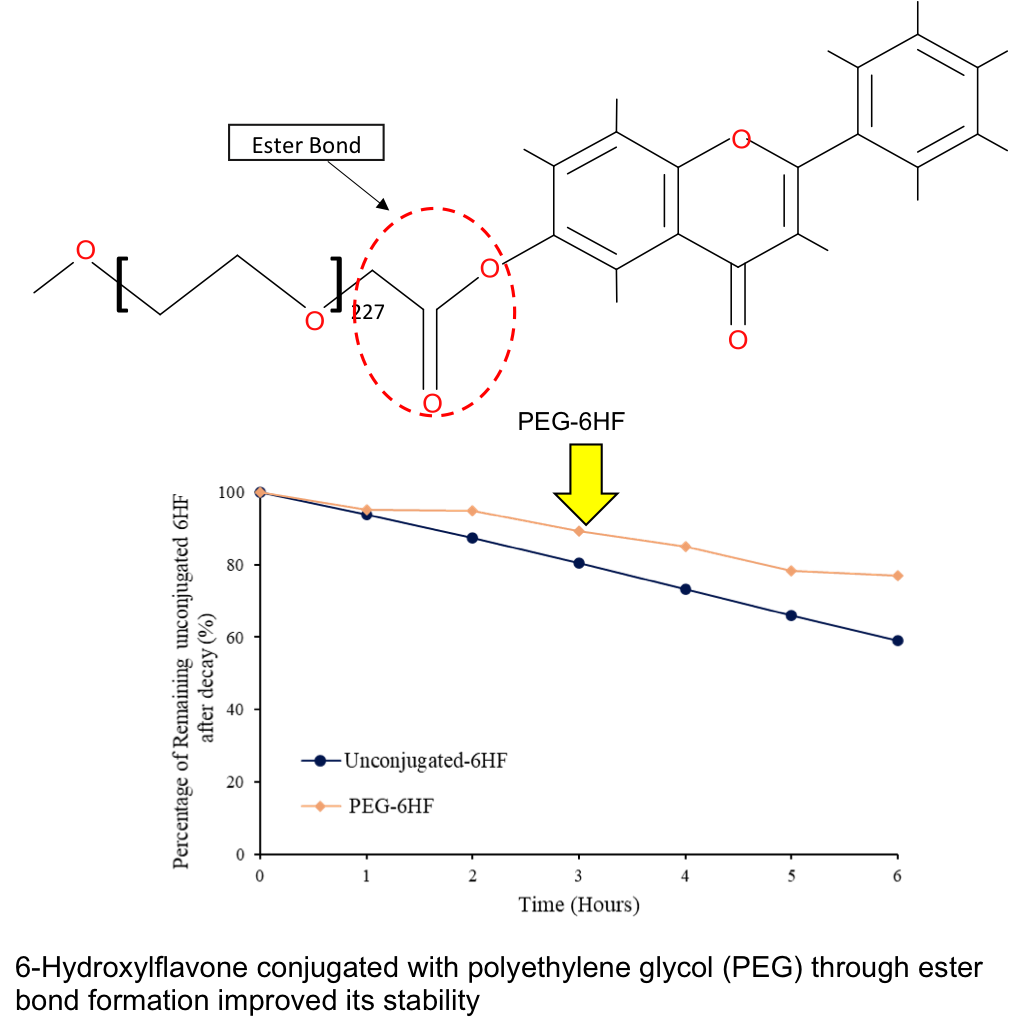
6-Hydroxyflavone (6HF) contains
attractive biological properties of significance pharmacologically and has been
discovered as an effective diabetic medication. However, due to its high
degradation in aqueous solution, its efficacy in biological treatment remains a
considerable obstacle. Thus, conjugating a polymer, polyethylene-glycol, to 6HF
by direct esterification between the carboxyl group of PEG and the hydroxyl
group at the sixth carbon of the 6HF biomolecule is one of the approaches
applied in this research to increase its stability while maintaining the inherent
biological characteristics. This
study examined the optimum esterification reaction conditions for conjugate
PEG-6HF utilizing EDC and DMAP as conjugation reagents in various solvents,
such as DMSO, PBS, and PBS 10 mM HEPES pH7.4 with the assistance of HOBt,
including its stability in the biomimicking environment. For this purpose,
PEG-6HF connected through the ester bond was validated using various analytical
techniques such as FTIR, UV-Vis spectroscopy, and HPLC. , Notably,
esterification at 25 °C for 24 hours in a 10 mM HEPES pH 7.4 buffer solution
using EDC with HOBt resulted in the most significant conjugation percentage, 42
percent. Furthermore, PEG-6HF revealed 1.3 times lower degradation percentages
of 6HF biomolecules than unconjugated-6HF following 6 hours of incubation in 10
mM HEPES pH 7.4 at 37 °C. Hence, the optimal conditions and the resulting
conjugation percentage with high stability are expected to be a fundamental
approach to conjugated polymer with a biomolecule.
Conjugation; Esterification; 6-Hydroxyflavone; PEG-6HF; Stability
Recently, a breakthrough has emerged in polymer
chemistry development by applying a fundamental theory of polymer phase
behavior at site-specific modification of peptides, synthetic biology, and
single-chain polymer behavior (Shu et al., 2013; Dey et al., 2015; Krisanti et al., 2020).
However, there have been slight changes in obtaining successful selective
molecular transport, hierarchical structure control, modulated responsiveness
to small perturbation, and long-term enzymatic activity (Shu et al., 2013).
Therefore, researchers have agreed that polymer-biomolecule conjugate would
improve this limitation. Currently, few conjugation methods have been discovered,
for example, click chemistry, amidation, thiol-maleimide, and esterification (Shimokawa et al., 2009; Liechty et al., 2010; Shu et al., 2013; Che-Harun et al., 2016).
Nevertheless, esterification is seen as a better alternative due to its simplicity,
eliminating the need for further biomolecule modification. Therefore, polymer-biomolecule conjugates
formed by direct covalent conjugation through the esterification process are a
new class of soft materials since each component is complementary (Shu
et al., 2013; Dey et al., 2015).
6-Hydroxyflavone (6HF) is a group of naturally derived
bioactive polyphenolic compounds that possess tremendous medicinal assets that
have potential roles in preventing chronic diseases, including effectiveness
against some neurological disorders paraplegia or sciatica (Wang
et al., 2021).
6HF has pharmacologically significant biological characteristics such as
neuroprotection, antimicrobial, anti-inflammatory, anticancer, and antioxidants
and has been discovered to be an effective treatment for diabetic patients
against glomerulonephritis and glomerulosclerosis (Iwakiri
et al., 2013;
Wang et al., 2015; Das
et al., 2018;
Das et al., 2019;
Stompor et al., 2019; Wang
et al., 2021).
Stompor
et al. (2019) and
Mikell
et al. (2015)
revealed that the hydroxyl group or the propionyl group located in the A ring
of the flavones at the C-6 positions has an inhibitory effect on hormone
production in the process of steroidogenesis and has cytotoxic solid and
apoptotic activities against cancer cell (Iwakiri
et al., 2013; Mikell
et al., 2015;
Wang et al., 2015;
Stompor et al., 2019). A hydroxyl group at the sixth carbon in the
6HF compound chemical structure makes 6HF easily esterifiable. Even though much
research focuses on intramolecular chemical changes of 6HF for certain specific
applications, the stability of 6HF and its derivatives in an aqueous solution
remains a big challenge.
Previously, Bayard
et al. (2013) reported
the successful conjugation between PEG with low molecular weight hydrophobic
biomolecules, including hormones and antioxidants, through esterification leads
to excellent pharmacokinetic properties of the drug. The objective of
developing PEG conjugates was to enhance water solubility and stability while
also lessening clearance through the kidney, which prolonged circulation in the
bloodstream and increased the drug molecule's biocompatibility (Bayard et al.,
2013; Hamley, 2014; Cui et al., 2021). Additionally, PEG has good
solubility and stability, increasing membrane permeability and enhancing oil
recovery (Febriasari
et al., 2021; Irawan et al., 2017). Furthermore, PEG is appropriate
for biological applications due to its biological inertness and low toxicity (Rashmi et al.,
2020; Turecek & Siekmann, 2019). Moreover, there has been no
fact-finding discussion on the conjugation of a 6HF biomolecule with a polymer
unit to the authors' knowledge. Thus, researchers were inspired to direct
conjugate a molecule of 6HF with a hydrophilic biodegradable polymer,
polyethylene glycol (PEG), to improve 6HF stability. Researchers investigated
the optimum esterification conditions of a PEG having a carboxyl functional
end-group with a 6HF molecule with an active hydroxyl functional group using
various solvents such as 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
(HEPES), dimethyl sulfoxide (DMSO), and phosphate buffer saline (PBS) to expand
the compatibility of the conjugation process and evaluated PEG—6HF conjugates
stability in the biomimicking environment. Figure 1 depicts the general route
for the conjugation of PEG-6HF. The produced PEG-6HF conjugates were characterized
by their physicochemical properties by RP-HPLC analysis, FTIR, and UV-Vis
analysis. The results revealed that the conjugation of PEG-6HF in 10 mM HEPES
with pH 7.4 buffer solvent with the assistance of additive peptide coupling,
HoBt, demonstrated the highest percentage of conjugation. This is the first
research to develop a conjugation between a 6HF biomolecule with a unit of
polymer and the conjugate enhanced 6HF stability in a biological environment.
Moreover, this research analyzes the optimum conjugation conditions of the PEG
and 6HF through esterification, which may aid in understanding the new molecular construction, which is expected to
be a new methodology for material chemistry research to provide crucial
information for future bioorganic and medicinal chemistry studies.
Figure 1 Synthetic scheme of PEG-6HF
In conclusion, a successful
conjugation with a high percentage yield (42 percent) between polyethylene
glycol (PEG) and 6-Hydroxyflavone (6HF) synthesized by direct esterification in
10 mM HEPES with pH 7.4 at 25°C was
observed in comparison to the PEG-6HF conjugate that prepared in DMSO (11
percent). Moreover, the PEG-6HF conjugate significantly improved its stability,
which was 20 percent more stable than unconjugated-6HF due to 1.3 times lower
degradation in physiological conditions for 6 hours. The antimicrobial activity
of PEG-6HF conjugate is currently being studied. Hence, this new conjugation
can be a fundamental methodology for
material chemistry field study and provide critical information for future bioorganic and medicinal chemistry studies.
The
authors thank the Ministry of Higher Education (MOHE) Malaysia for the
financial support provided via the Fundamental Research Grant Scheme [ref no.
FRGS/1/2018/STG05/UNIKL/02/5]. The authors also thank Universiti Kuala Lumpur
Malaysian Institute of Chemical and Bioengineering Technology, Melaka,
Malaysia, for providing resources and necessary facilities for FTIR, UV-Vis
Spectrophotometer, and HPLC analyses.
Bayard, F.J.C., Thielemans, W., Pritchard, D.I., Paine, S.W.,
Young, S.S., Bäckman, P., Ewing, P., Bosquillon, C., 2013. Polyethylene Glycol-Drug
Ester Conjugates for Prolonged Retention of Small Inhaled Drugs in the Lung. Journal
of Controlled Release, Volume 171(2), pp. 234–240
Charoensit, P., Pompimon, W., Khorana, N., Sungthongjeen, S.,
2019. Effect of Amide Linkage of PEG-Lipid Conjugates on the Stability and
Cytotoxic Activity of Goniodiol Loaded in PEGylated Liposomes. Journal of
Drug Delivery Science and Technology, Volume 50, pp. 1–8
Che-Harun, N.F., Takemoto, H., Nomoto, T., Tomoda, K.,
Matsui, M., Nishiyama, N., 2016. Artificial Control of Gene Silencing Activity
Based on siRNA Conjugation with Polymeric Molecule Having Coil-Globule
Transition Behavior. Bioconjugate Chemistry, Volume 27(9), pp. 1961–1964
Cui, Z., Luo, Q., Bannon, M.S., Gray, V.P., Bloom, T.G.,
Clore, M.F., Hughes, M.A., Crawford, M.A., Letteri, R.A., 2021. Molecular Engineering
of Antimicrobial Peptide (AMP)-Polymer Conjugates. Biomaterials Science,
Volume 9(15), pp. 5069–5091
Das, S., Karn, A., Sarmah, R., Rohman, M.A., Koley, S.,
Ghosh, P., Roy, A.S., 2018. Characterization of Non-Covalent Binding of 6-Hydroxyflavone
and 5,7-Hydroxyflavone with Bovine Hemoglobin: Multi-Spectroscopic and Molecular
Docking Analyses. Journal of Photochemistry and Photobiology B: Biology,
Volume 178, pp. 40–52
Das, S., Santra, S., Rohman, M.A., Ray, M., Jana, M., Roy, A.
S., 2019. An Insight into the Binding of 6-Hydroxyflavone with Hen Egg-White Lysozyme:
A Combined Approach of Multi-Spectroscopic and Computational Studies. International
Journal of Biomolecular Structure and Dynamics, Volume 37 (15), pp.
4019–4034
Dey, S., Ambattu, L.A., Hari, P.R., Rekha, M.R., Sreenivasan,
K., 2015. Glutathione-Bearing Fluorescent Polymer-Curcumin Conjugate Enables Simultaneous
Drug Delivery and Label-Free Cellular Imaging. Polymer, Volume 75, pp.
25–33
Esmaeili, Y., Bidram, E., Zarrabi, A., Amini, A., Cheng, C., 2020.
Graphene Oxide and its Derivatives as Promising In-Vitro Bio-Imaging Platforms.
Scientific Reports, Volume 10(1), pp. 1–13
Febriasari, A., Suhartini, M., Yunus, A.L., Rahmawati, R.,
Sudirman, S., Hotimah, B., Hermana, R.F., Kartohardjono, S., Fahira, A., Permatasari,
I.P., 2021. Gamma Irradiation of Cellulose Acetate-Polyethylene Glycol 400
Composite Membrane and Its Performance Test for Gas Separation. International
Journal of Technology, Volume 12(6), pp. 1198–1206
Hamley, I.W., 2014. PEG-Peptide Conjugates. Biomacromolecules,
Volume 15(5), pp. 1543–1559
Irawan, Y., Juliana, I., Adilina, I.B., Alli, Y. F., 2017.
Aqueous Stability Studies of Polyethylene Glycol and Oleic Acid-Based Anionic Surfactants
for Application in Enhanced oil Recovery through Dynamic Light Scattering. International
Journal of Technology, Volume 8(8), pp. 1414–1421
Iwakiri, T., Mase, S., Murakami, T., Matsumoto, M., Hamada,
H., Nakayama, T., Ozaki, S.I., 2013. Glucosylation of Hydroxyflavones by Glucosyltransferases
from Phytolacca Americana. Journal of Molecular Catalysis B: Enzymatic,
Volume 90, pp. 61–65
Krisanti, E.A., Lazuardi, D., Kiresya, K.K., Mulia, K.,2020.
Tablet Formulation Containing Chitosan-Alginate Microparticles:
Characterization and Release Profile of Xanthones. International Journal of
Technology, Volume 11(5), pp. 900–909
Liechty, W.B., Kryscio, D.R., Slaughter, B.V., Peppas, N.A., 2010.
Polymers for Drug Delivery Systems. Annual Review of Chemical and
Biomolecular Engineering, Volume 1(1), pp. 149–173
Liu, Y., Yang, L., Guo, Y., Zhang, T., Qiao, X., Wang, J.,
Xu, J., Xue, C., 2020. Hydrophilic Astaxanthin: PEGylated Astaxanthin Fights
Diabetes by Enhancing the Solubility and Oral Absorbability. Journal of
Agricultural and Food Chemistry, Volume 68(11), pp. 3649–3655
Mikell, J.R., Herath, W., Khan, I.A., 2015. Eleven Microbial Metabolites
of 6-Hydroxyflavanone. Chemical and Pharmaceutical Bulletin, Volume
63(8), pp. 579–583
Rashmi, Zabihi, F., Singh, A.K., Achazi, K., Schade, B.,
Hedtrich, S., Haag, R., Sharma, S.K. , 2020. Non-Ionic PEG-Oligoglycerol Dendron
Conjugated Nano-Carriers for Dermal Drug Delivery. International Journal of
Pharmaceutics, Volume 580, p. 119212
Shimokawa, K., Yamada, K., Ohno, O., Oba, Y., Uemura, D., 2009.
Design, Synthesis, and Biological Evaluation of Biotin-Labeled (-)-Ternatin, a Potent Fat-Accumulation Inhibitor Against
3T3-L1 Adipocytes. Bioorganic and Medicinal Chemistry Letters, Volume
19(1), pp. 92–95
Shu, J.Y.,
Panganiban, B., Xu, T., 2013. Peptide-Polymer Conjugates: From Fundamental
Science to Application. Annual Review of Physical Chemistry, Volume
64(1), pp. 631–657
Stompor, M., Switalska, M., Bajek, A., Wietrzyk, J., 2019.
Influence of Amide Versus Ester Linkages on the Anticancer Properties of the New
Flavone-Biotin Conjugates. Zeitschrift Fur Naturforschung - Section C
Journal of Biosciences, Volume 74(7–8), pp. 193–200
Ta-Aithuak, S., Loedsapchinda, N., Houngkamhang, N., 2020.
Conjugation of Antibody on Gold Nanoparticles for Biosensors Application. Key
Engineering Materials, Volume 853 KEM, pp. 92–96.
Turecek, P.L., Siekmann, J., 2019. PEG-Protein Conjugates:
Nonclinical and Clinical Toxicity Considerations. In: Polymer-Protein
Conjugates, Pegylation and Beyond, Elsevier B.V., pp. 61–101
Wang, X., Cao, Y., Chen, S., Lin, J., Bian, J., Huang, D., 2021.
Anti-Inflammation Activity of Flavones and Their Structure-Activity
Relationship. Journal of Agricultural and Food Chemistry, Volume 69(26),
pp. 7285–7302
Wang, X., Wang, Z., Sidhu, P.S., Desai, U.R., Zhou, Q., 2015.
6-Hydroxyflavone and Derivatives Exhibit Potent Anti-Inflammatory Activity
Among Mono-, Di- and Polyhydroxylated Flavones in Kidney Mesangial Cells. PLoS
ONE, Volume 10(3), pp. 1–11
