Synthesis and Characterization of Cellulose Ethers from Screw Pine (Pandanus tectorius) Leaves Cellulose as Food Additives
Corresponding email: venty@mipa.uns.ac.id
Published at : 09 May 2023
Volume : IJtech
Vol 14, No 3 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i3.5288
Suryanti, V., Kusumaningsih, T., Safriyani, D., Cahyani, I.S., 2023. Synthesis and Characterization of Cellulose Ethers from Screw Pine (Pandanus tectorius) Leaves Cellulose as Food Additives. International Journal of Technology. Volume 14(3), pp. 659-668
| Venty Suryanti | 1. Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sebelas Maret, Jl. Ir. Sutami 36 A Surakarta 57126, Indonesia, 2. Center for Research and Development of Biotechn |
| Triana Kusumaningsih | Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sebelas Maret, Jl. Ir. Sutami 36 A Surakarta 57126, Indonesia |
| Diah Safriyani | Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sebelas Maret, Jl. Ir. Sutami 36 A Surakarta 57126, Indonesia |
| Indri Sri Cahyani | Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sebelas Maret, Jl. Ir. Sutami 36 A Surakarta 57126, Indonesia |
Isolation
of
cellulose from Screw pine (Pandanus
tectorius) leaves and its transformation into cellulose ethers have been
successfully conducted. Crystalline cellulose was obtained as a white powder in
40.24% yield from dried Screw pine leaf powder through alkalization with sodium
hydroxide followed by bleaching with sodium hypochlorite. The crystalline
cellulose was converted to methyl cellulose (MC), carboxymethyl cellulose
(CMC), and hydroxypropyl cellulose (HPC) and
obtained 94.6%, 71.47%, and 81.54% yields, respectively. The
physicochemical properties of MC, CMC, and HPC, such as shape, color, pH,
Degree of Substitution (DS), and viscosity meet the standards for application
as a food additive. The pH and viscosity of the synthetic cellulose ethers were
7.53-7.60 and 61.98-62.41 cP, respectively. The DS value for MC,
CMC, and HPC were 1.680, 0.996, and 0.696, respectively. The study
revealed the potential of Screw pine cellulose to obtain food-grade cellulose
ethers since the properties of MC, CMC, and HPC agree with food additive
criteria.
Cellulose; Cellulose ethers; Food additive; Pandanus tectorius; Screw pine
Food additives are often added during food
processing to improve the taste, flavor, color, texture, and nutritional value.
Food additives are also used as preservatives for longer storage. The
consumption of food additives, especially synthetic additives, can cause
long-term negative health effects (Sahu, 2017).
Therefore, there is a great interest in the use of food additives from natural
sources to minimize the side effects. Demand for functional food has been
growing globally (Suryanti et al., 2021; 2020;
2016). Biopolymer-based materials from polysaccharide, especially
cellulose has great potential to be used as food additives (Li et al., 2018). In particular, cellulose
derivatives are widely used as food since they have low viscosity which adds
new properties to food products.
Cellulose can be obtained from
natural resources by acid-alkali treatment. The production of cellulose and its
derivatives from raw materials that are economically cheaper and locally
available is desirable. Cellulose is commonly converted into useful derivatives
by reactions typical of compounds containing hydroxyl groups. The hydroxyl
groups (-OH) of cellulose can be partially or fully reacted with
various reagents to obtain cellulose
ethers with useful properties (Tosh, 2014).
The isolation of cellulose ethers in living organisms, such as plants, animals,
bacteria, fungi, and yeast, is not reported yet. Therefore, synthetic cellulose
ether needs to be developed. Etherification of cellulose changes cellulose’s
solubility significantly, where cellulose ethers dissolve in water, dilute
acids, dilute alkalis, or act as organic solvents. Cellulose ethers, such
as methyl cellulose (MC), carboxymethyl
cellulose (CMC) and hydroxypropyl cellulose (HPC), have demonstrated useful
applications in various areas including food, personal care products, oil-field
chemicals, construction materials, the paper industry, as well as adhesives and
textile industries (Kimani et al., 2016;
Bhatt et al., 2011).
2.1. Materials and Methods
The
Screw pine leaves were obtained from Gunung Kidul coastal area, Yogyakarta,
Indonesia. All chemicals were purchased from Sigma-Aldrich (Germany) and used
without any further purification. Fourier Transform Infrared (FT-IR) spectrum
was obtained by SHIMADZU FT-IR-prestige 21 using a KBr pellet in the range of
4000–400 cm-1. The powder was characterized by X-Ray diffraction
XPert MPD using Cu–K radiation (
= 1.5406 Å; 2
= 5–60°) at the voltage of 40
kV and current at 30 mA. Scanning Electron Microscope (SEM) data were collected
by SEM JSM-6510 with voltage at 10 kV and pressure at 70 Pa. Thermogravimetric
Analysis (TGA) of STA Linseis pt 1600 was used where the sample was heated to 600? at a heating rate of 10oC/min under atmospheric
pressure.
2.2.
Isolation of Cellulose from Screw Pine Leaves
The isolated cellulose was then analyzed by Fourier Transform Infra-Red (FTIR) spectroscopy, Scanning Electron Microscopy (SEM), Thermogravimetric Analysis (TGA), and X-ray Diffraction (XRD).
2.3.
Synthesis of Methyl Cellulose (MC)
MC was synthesized following Viera et al. (2007) method with some modifications. Screw pine leaf cellulose (1 g) was added to a mixture of 50% NaOH (5 mL) and isopropanol (30 mL). The mixture was stirred for 1 h at room temperature. The mixture was then filtered and the residue was mixed with acetone in a ratio of 1:20. The mixture was refluxed for 6 h at 50oC with dichloromethane being added dropwise in a ratio of 1:10. The mixture was neutralized with 10% acetic acid and then washed with 95% ethanol. The residue was dried at 50? to obtain pure MC.
2.4.
Synthesis of Methyl Cellulose (MC)
CMC was synthesized from Screw pine leaves cellulose according to Parid et al. (2018) method with some modifications. The CMC synthesis was performed in two steps: alkalization and etherification of cellulose. In the alkalization reaction, 1 g of cellulose was stirred in a mixture of 3 mL of 20% NaOH and 20 mL isopropanol for 1 h at room temperature. For the etherification reaction, the mixture was heated to 50oC and gradually added with a solution of trichloroacetic acid/isopropanol (1 g: 4 mL) and further stirred for 3 h. The resulting slurry was filtered and neutralized with 10% acetic acid. Then, the residue was rinsed with 95% ethanol to remove undesired by products. The residue was dried at 60oC for 6 h to obtain pure CMC.
2.5.
Synthesis of Hydroxypropyl cellulose (HPC)
HPC was synthesized according to Marseno et al. (2014) method with modifications. Screw pine cellulose (1 g) was added and mixed with 10% NaOH (4 mL) and isopropanol (25 mL). The mixture was stirred for 1 h at room temperature. The mixture was then refluxed for 3 h at 55oC where propylene oxide (1.4 mL) was added in a dropwise. The resulting mixture was filtered and the residue was neutralized with 10% acetic acid and then washed with 95% ethanol. The residue was dried at 60oC for 6 h to obtain pure HPC.
2.6.
Characterization of MC, CMC, and HPC
MC, CMC, and HPC were also measured for pH, degree of substitution
(DS), and viscosity. The cellulose ethers were analyzed by Fourier Transform Infra-Red
(FTIR) Spectroscopy and Scanning Electron Microscopy (SEM).
2.7. Instruments
The
functional groups of the cellulose and cellulose ethers were determined using a
Shimadzu FTIR Prestige 21 model 8201 PC. The surface morphologies of samples were examined by SEM
(JEOL JSM- 6510LA). The
thermal stability of cellulose was studied by thermogravimetric analysis using
an STA Linseis pt 1600 TGA analyzer. The degree of crystallinity of cellulose
was determined using an X-Ray diffractometer (XRD XPert MPD diffractometer).
3.1. Isolation of Cellulose
Cellulose was isolated from Screw
pine leaves by alkalization and bleaching processes. The white cellulose was
obtained in a 40.24% (w/w) yield. FTIR spectrum of isolated cellulose is
consistent with the results reported by Sheltami et
al. (2012). Obtained SEM image showed a typical shape of cellulose.
Cellulose fibers were individually dispersed which were separated and elongated
like ribbons with approximate diameters of 5.97 in the
range of 1.5–17
. The cellulose fibers' size is longer than
that of Screw pine leaf fibers. These results are consistent with research
conducted by Sheltami et al. (2012).
Thermogravimetric analysis of purified cellulose showed that thermal
degradation of cellulose occurred in the range 205-407oC with a significant
weight loss of 86.52%. The X-Ray diffractogram revealed that cellulose
structure was categorized as a crystal I type (Sheltami
et al., 2012). The crystallinity index of the isolated cellulose
was 83.03% which is higher than the value reported for the cellulose of rice
straw at 69.5% (Sheltami et al., 2012)
and bagasse cellulose at 58.77% (Nu et al., 2019). The data obtained from FT-IR, SEM, TGA, and X-Ray
Crystallography analyses confirmed that cellulose has been obtained from Screw
pine leaves.
3.2. Synthesis of Cellulose Ethers from Cellulose
of Screw pine (Pandanus tectorius) Leaf
Williamson's etherification reaction was
used to synthesize the MC, CMC, and HPC. Williamson ether synthesis utilizes
deprotonated alcohol and an organohalide to form an ether. The alkoxide ion is a good
nucleophile and displaces halide ions from the alkyl halide to give a new
carbon oxygen bond. An alkoxide is produced by the treatment of alcohol with
either a base or an alkali metal. In this reaction, sodium hydroxide is reacted
with cellulose to obtain Na-cellulose.
The initial step of cellulose ether synthesis was performed by stirring screw
pine leaf cellulose and sodium hydroxide, forming a yellow-brown mass.
In the synthesis of MC, the
etherification step was carried out by the addition of dichloromethane to
Na-cellulose. The MC was obtained as white powders in 94.61% yield. The synthesis of CMC was
accomplished by reacting Na-cellulose and trichloroacetic acid (TCA). CMC is
commonly prepared by the reaction of cellulose with monochloroacetic acid
(MCA). In this work, TCA was used instead of MCA. The CMC was obtained as a white
powder with a 71.47% yield. This yield is double compared to that reported by Bono et al. (2009) (33.15%) for CMC from
palm kernel cake; and Huang et al. (2017) (64.40%)
for CMC from spent tea leaf. The difference in yield could be attributed to the
temperature of the reaction, and the concentration of NaOH and
carboxymethylating agent. The synthesis of HPC was conducted by mixing
Na-cellulose and propylene oxide at 55°C. The HPC was obtained as white powders
in 81.54% yield. The highest yield was obtained for the synthesis of MC may due
to the steric reason where the methyl group caused the least steric bulk.
3.3. FT-IR Spectral Analysis of Cellulose Ethers
FT-IR spectra of the cellulose ethers
confirm the successful synthesis of MC, CMC, and HPC from Screw pine cellulose
(Figure 2 and Table 1). There are typical absorption peaks of MC at 1500-800 cm-1
(Ibrahim et al., 2015; Viera et al.,
2007). An absorption of -CH alkane appears at 1414 cm-1
indicating the presence of methyl compounds of MC. Additionally, the presence
of a peak at 1158 cm-1 for C-O ether and a peak at 1574 cm-1 peak
for C-C confirmed that MC was obtained. The typical cellulose peaks are also
found at 3389-3445 cm-1 for -OH groups, at 2902 cm-1
for -CH (stretching) groups, at 1339 cm-1 for -CH (bending) groups,
at 1022 and 1062 cm-1 for carbonyl (C=O) group (stretching) and at
895-927 cm-1 for -CH uptake (rocking) deformation of the
1,4-glycosidic bond.
Figure 2 FTIR spectra of cellulose (a), MC (b), CMC (c), and HPC (d)
Table 1 FTIR spectra analysis of
cellulose, MC, CMC, and HPC
|
Absorption band (cm-1) |
Functional Groups | |||
|
Cellulose |
MC |
CMC |
HPC | |
|
3384 |
3389-3445 |
3445 |
3445 |
-OH
stretching |
|
2902 |
2902 |
2899 |
2899 |
-CH
stretching of methyl and methylene |
|
- |
- |
- |
1650 |
OH bending (water) |
|
- |
- |
1570-1638 |
- |
-C=O
stretching |
|
1431 |
- |
1416 |
1416 |
-CH2
and -CH3 bending |
|
- |
1317-1414 |
- |
- |
-CH2,
-CH3 scissoring |
|
1321 |
- |
1330 |
- |
C-O
stretching ether group |
|
1112-1163 |
1158 |
1159 |
1159 |
C-O-C
stretching asymmetric |
|
1031-1058 |
1022-1062 |
1024-1066 |
1063 |
O-C-O
stretching of ether |
|
898 |
896 |
895 |
896 |
C-H
bending ( |
The FTIR spectra of
HPC reveal the presence of functional groups of HPC (Joshi
et al., 2019; Marseno et al., 2014; Bhatt et al., 2011;
Sharma et al., 2009). Absorption at 2899 cm-1 and 1416
cm-1 shows C-H and methylene groups of hydroxypropyl cellulose,
respectively. In addition, there is also absorption at 1063 and 1159 cm-1 for
C-O ether (stretching). The typical absorption of cellulose was found at 3445
cm-1 for absorption of the -OH group (stretching), 1323 cm-1
for the -CH group (bending), and 896 cm-1 for absorption of -CH
(stretching) deformation of the 1,4-glycosidic bond. This indicates that
cellulose was successfully modified into HPC.
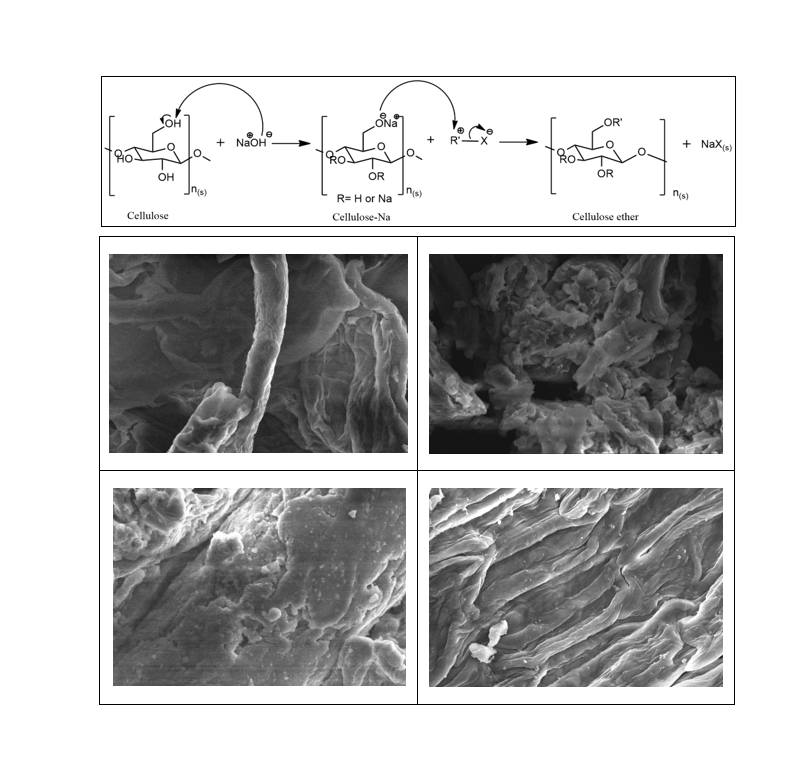
3.4. SEM Analysis of MC, CMC, and HPC
Figure 3 SEM micrograph of
cellulose (a), MC (b), CMC (c), and HPC (d) at magnification
3000X
3.5. Physical Properties of Cellulose Ethers
The physical properties of cellulose
ethers are presented in Table 2. DS is one of the important parameters in
determining the quality of cellulose ethers. DS is the average number of hydroxyl groups
substituted in every anhydroglucose unit in the chain. The higher the DS value of the product obtained, the better the
cellulose ether quality. DS of cellulose ethers was found to be
dependent upon the source of cellulose, therefore cellulose ethers prepared
from various cellulose sources may have various DS values.
The DS of the MC product was 1.68 which
is in the range of 1.3-2.6 for the DS value of MC as a food additive. The commercial DS of CMC is
0.5-1.5 (Karatas and Arslan, 2016). CMC
having DS below 0.4 is swellable but insoluble in water. CMC having DS above
0.4 is soluble in water, because the hydro-affinity of CMC is enhanced with
increasing DS value (Varshney et al., 2006).
The DS value of synthesized CMC was 0.996, indicating it is soluble in water.
The result is higher than the DS of CMC from palm kernel cake (0.31) and oil
palm fiber (0.29) (Huang et al., 2017).
DS values of CMC from Miscanthus sinensis,
Eichhornia crassipes, and Cyperus papyrus are 0.35, 0.80, and
0.76, respectively (Kimani et al. 2016).
The DS of synthesized HPC was 0.696. This result is in accordance with the
characteristics of HPC as a food additive where the DS value of HPC should be
above 0.12.
The pH of the 1%
solution of MC, CMC, and HPC in distilled water were 7.53, 7.60, and 7.56, respectively. The
pH of food grade MC, CMC, and HPC according to FAO standards are 5-8, 6-8.5, and 5-8.5, respectively.
These results suggest that the pH of the synthesized MC, CMC, and HPC meet the
standards as food additives.
The viscosity value of 1% MC, CMC, and
HPC solution in distilled water was 62.39; 62.41, and 61.98 cP, respectively. These results are in accordance with the MC,
CMC, and HPC standards as food additives. The viscosity of CMC is correlated
with DS value: a higher DS results in higher cation exchangeability and
viscosity. The low DS value indicates fewer hydrophilic groups, which implies
the reduction of the bonding of polymer with water molecules. Higher viscosity
(66.6 cP) was reported for CMC from Palm Kernel Cake (PKC) (Bono et al., 2009) while lower viscosity
(14.0 cP) was reported for CMC from orange mesocarp. The variation in
properties of the different CMC could be a result of the source of cellulose
used, plant species, age, and source which affect the cellulose content
compositions (Carere et al., 2008).
Table 2 Physicochemical properties of MC, CMC, and HPC
|
Properties |
Cellulose Ethers | ||
|
MC |
CMC |
HPC | |
|
Physical
state and Appearance |
white
powder |
white
powder |
white
powder |
|
pH |
7.53 |
7.60 |
7.56 |
|
Viscosity
|
62.39
cP |
62.41
cP |
61.98
cP |
|
DS |
1.680 |
0.996 |
0.696 |
Cellulose was isolated from Screw pine (Pandanus tectorius) leaves in 40.24 % yield employing alkali
treatment followed by bleaching with sodium hypochlorite. The structure of the
obtained cellulose was confirmed by FTIR, SEM, and TGA analysis. Cellulose ethers
have been successfully synthesized from Screw pine leaves cellulose through
Williamson ether synthesis. Cellulose ethers MC, CMC, and HPC were obtained in
94.6%, 71.47%, and 81.54% yields, respectively. The products have been
confirmed by FTIR spectra and SEM images. The physicochemical properties, such
as shape, color, pH, viscosity, and DS suggest that synthesized MC, CMC, and
HPC meet the criteria as food additives.
The grant research with contract number 452/UN27.21/PN/2020 from Sebelas
Maret University, Indonesia is greatly appreciated.
Afolabi, L.O., Megat-Yusoff, P.S.M., Ariff, Z.M., Hamizol, M.S.,
2019. Fabrication of Pandanus Tectorius
(Screw-Pine) Natural Fiber Using Vacuum Resin Infusion for Polymer Composite
Application. Journal of Materials Research and Technology, Volume 8(3), pp. 3102–3113
Alabi, F.M., Lajide, L., Ajayi, O.O.,
Adebayo, A.O., Emmanuel, S., Fadeyi, A.E., 2020. Synthesis And Characterization
of Carboxymethyl Cellulose from Musa Paradisiaca
And Tithonia Diversifolia. African
Journal of Pure and Applied Chemistry, Volume 14(1), pp. 9–23
Bhatt, N., Gupta, P.K., Naithani, S.,
2011. Hydroxypropyl Cellulose From A-Cellulose Isolated from Lantana Camara
with Respect To DS And Rheological Behaviour. Carbohydrate Polymers,
Volume 86, pp. 1519–1524
Bono, A., Ying, P.H., Yan, F.Y., Muei,
C.L., Sarbatly, R, Krishnaiah, D., 2009. Synthesis and Characterization of
Carboxymethyl Cellulose From Palm Kernel Cake. Advances in Natural and Applied
Sciences, Volume 3(1),
pp. 5–12
Carere, C.R., Sparling, R., Cicek, N.,
Levin, D.B., 2008. Third Generation Biofuels Via Direct Cellulose Fermentation.
International Journal of Molecular Sciences, Volume 9(7), pp. 1342–1360
Haleem, N., Arshad, M., Shahid, M., Tahir, M.A. 2014. Synthesis of Carboxymethyl
Cellulose from Waste Of Cotton Ginning Industry. Carbohydrate Polymers,
Volume 113, pp. 249–255
Huang, C.M., Chia, P., Lim, C.S., Nai, J., Ding, D.Y., Seow, P.,
Chan, E.W., 2017. Synthesis and Characterisation of Carboxymethyl Cellulose
From Various Agricultural Wastes. Cellulose Chemistry and Technology,
Volume 51, pp. 665–672
Ibrahim, M.M., Fahmy, T.Y., Salaheldin, E.I.,
Mobarak, F., Youssef, M.A., Mabrook, M.R., 2015. Synthesis of
Tosylated and
Trimethylsilylated Methyl Cellulose As Ph-Sensitive Carrier Matrix. Life
Science Journal, Volume 1(12), pp. 29–37
Joshi, G., Rana, V., Naithani, S.,
Varshney, V.K., Sharma, A., Rawat, J.S., 2019. Chemical Modification of Waste Paper: An
Optimization Towards Hydroxypropyl Cellulose Synthesis. Carbohydrate Polymers, Volume 223, pp. 1–12
Karatas, M., Arslan, N., 2016. Flow Behaviours of Cellulose And
Carboxymethyl Cellulose From Grapefruit Peel. Food Hydrocolloids,
Volume 58, pp. 235–245
Kimani, P.K., Kareru, P.G., Madivoli, S.E.,
Kairigo, P.K., Maina, E.G., Rechab, O.S., 2016. Comparative Study of
Carboxymethyl Cellulose Synthesis From Selected Kenyan Biomass. Chemical
Science International Journal, Volume 17(4), pp. 1–8
Li, Y.Y., Wang, B., Ma, M.G., Wang, A., 2018. Review of Recent
Development On Preparation, Properties, And Application Of Cellulose-Based
Functional Materials. International Journal of Polymer Science, Volume
2018, pp. 1–18
Li, W., Cai, G., Zhang, P., 2019. A Simple and Rapid Fourier
Transform Infrared Method For The Determination Of The Degree Of Acetyl
Substitution Of Cellulose Nanocrystals. Journal of Materials Science,
Volume 54(10), pp. 8047–8056
Nasatto, P.L., Pignon, F., Silveira, J.L.M., Duart, M.E.R., Noseda,
M.D., Rinaudo, M., 2015, Methylcellulose, a cellulose derivative with original
physical properties and extended applications, Polymers, Volume 7, pp.
777–803
Marseno, D.W., Haryanti, P., Adiseno, B.,
Haryadi, 2014, Synthesis and Characterization Of Hydroxypropylcellulose From
Oil Palm Empty Fruit Bunches (Elaeis Guineensis Jacq), Indonesian
Food and Nutrition Progress, Volume 13(1), pp. 24–30
Parid, D.M., Abd Rahman, N.A., Baharuddin,
A.S., Mohammed, M.A.P., Johari, A.M., Razak, S.Z.A., 2018. Synthesis and
Characterization of Carboxymethyl Cellulose from Oil Palm Empty Fruit Bunch
Stalk Fibres. BioResources, Volume 13(1), pp. 535–554
Sahu, S.C., 2017. Food additives. Food and Chemical Toxicology, Volume
107, pp. 529-529
Sharma, R., Varshney, V.K., Chauhan,
G.S., Naithani, S., Soni, P.L., 2009, Hydroxypropylation of Cellulose Isolated
from Bamboo (Dendrocalamus Strictus) With Respect To Hydroxypropoxyl
Content And Rheological Behavior Of The Hydroxypropyl Cellulose. Journal of
Applied Polymer Science, Volume 113, pp. 2450–2455
Sheltami, R.M., Abdullah, I., Ahmad, I.,
Dufresne, A., Kargarzadeh, H., 2012. Extraction of Cellulose Nanocrystals from
Mengkuang Leaves (Pandanus Tectorius). Carbohydrate
Polymers, Volume 88(2), pp.
772–779
Sun, J.X., Sun, X.F., Zhao, H., Sun, R.C.,
2004. Isolation and Characterization Of Cellulose From Sugarcane Bagasse. Polymer
Degradation and Stability, Volume 84(2),
pp. 331–339
Suriyatem, R.,
Noikang, N., Kankam, T., Jantanasakulwong, K., Leksawasdi, N., Phimolsiripol,
Y., Sommano, S.R., 2020. Physical Properties of Carboxymethyl Cellulose From
Palm Bunch And Bagasse Agricultural Wastes: Effect Of Delignification With
Hydrogen Peroxide. Polymers, Volume 12(7), p. 1505
Suryanti, V., Marliyana, S.D., Putri, H.E., 2016. Effect
of Germination On Antioxidant Activity, Total Phenolics, ?-carotene, Ascorbic
Acid And ?-Tocopherol Contents Of Lead Tree Sprouts (Leucaena Leucocephala
(lmk.) de Wit). International Food Research Journal, Volume 23(1),
pp. 167–172
Suryanti, V., Riyatun, Suharyana, Sutarno, Saputra,
O.A., 2020. Antioxidant activity and compound constituents of gamma-irradiated
black rice (Oryza sativa l.) var. cempo ireng indigenous of Indonesia. Biodiversitas, 2020,
Volume 21(9), pp. 4205–4212
Suryanti, V., Marliyana, S.D., Rohana, G.L., Trisnawati,
E.W., Widiyanti, 2021. Bioactive Compound Contents and Antioxidant
Activity of Fermented Lead Tree (Leucaena Leucocephala (lmk.) de Wit)
Seeds. Molekul, Volume 16(3), pp. 192–199
Nu, D.T.T., Hung, N.P., Hoang, V.C.,
Bruggen, B.V.D., 2019. Preparation of an Asymmetric Membrane from Sugarcane
Bagasse Using DMSO as Green Solvent. Applied Sciences. Volume 9(16),
p. 3347
Tosh, B., 2014. Synthesis And Sustainable
Applications Of Cellulose Esters And Ethers:
A Review. International Journal of Energy, Sustainability and
Environmental Engineering, Volume 1(2), pp. 56–78
Viera, R.G.P., Filho, G.R., Assuncao,
R.M.N., Meirele, C.S., Vieira, J.G., Oliveira, G.S., 2007. Synthesis and Characterization
Of Methylcellulose From Sugar Cane Bagasse Cellulose. Carbohydrate Polymers,
Volume 67, pp. 182–185
Varshney, V.K., Gupta, P.K., Naithani, S., Khullar, R., Bhatt, A.,
Soni, P.L., 2006. Carboxymethylation of -cellulose Isolated from Lantana Camara With Respect To Degree Of
Substitution And Rheological Behaviour. Carbohydrate Polymers,
Volume 63(1), pp. 40–45
