Tobacco Extract for Inhibition of Carbon Steel Corrosion in H2S-contained NaCl Solution
Corresponding email: pramujo@che.itb.ac.id
Published at : 28 Jul 2023
Volume : IJtech
Vol 14, No 5 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i5.5272
Devianto, H., Nurdin, I., Widiatmoko, P., Silvia, D., Prakarsa, C., 2023. Tobacco Extract for Inhibition of Carbon Steel Corrosion in H2S-contained NaCl Solution. International Journal of Technology. Volume 14(5), pp. 1167-1176
| Hary Devianto | Chemical Engineering Program, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132 Indonesia |
| Isdiriayani Nurdin | Chemical Engineering Program, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132 Indonesia |
| Pramujo Widiatmoko | Chemical Engineering Program, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132 Indonesia |
| Della Silvia | Chemical Engineering Program, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132 Indonesia |
| Clarissa Prakarsa | Chemical Engineering Program, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132 Indonesia |
This study explored the effectiveness of tobacco
extract as a corrosion inhibitor for carbon steel in an H2S-containing
NaCl solution. The experiments were conducted at room temperature and
atmospheric pressure, with a variation in inhibitor concentration and rate of
fluid flow. Results showed that the tobacco extract decreases the corrosion
rate of carbon steel in the 3.5% NaCl solution. Increasing flow rate and
inhibitor concentration significantly affect the corrosion rate of carbon
steel. In the NaCl solution, crystalline corrosion product formation of
magnetite (Fe3O4) was mainly detected. Furthermore, the
presence of H2S leads to forming of a mackinawite (FeS) structure.
The inhibition efficiency of 1000 and 2000 ppm tobacco extract in NaCl solution
with the presence of H2S is in the range of 24 to 69%.
Carbon Steel; Corrosion; Flow rate; Hydrogen sulphide; Inhibitor; Tobacco extract
Chemical industries
produce daily necessities as a vital part of human life. The involvement of
corrosive fluid in chemical processing, both in static and dynamic flow, leads
to corrosion of the process equipment. Corrosion is a spontaneous metal deterioration
process due to environmental interaction (Roberge,
2008). In industries, carbon
steel is common material widely used for various purposes (Kurniawan, Mitha, and Fikri, 2018) because of its
satisfactory performance and attractive cost (Gandy,
2007). However, carbon steel is susceptible to corrosion (Dwivedi, Lepkova, and Becker, 2017).
Components such as sodium chloride and hydrogen sulfide in oil and gas
industries (Al-Janabi, 2020) increase the
corrosivity of process fluids to the carbon steel. The corroded carbon steel
leads to equipment failures, loss of containment, product contamination, and
work accident. Therefore, a study on carbon steel corrosion in NaCl and H2S
environments is crucial, especially in the petroleum industry, to reduce costly
social, economic, and even human losses (Zúñiga et
al., 2011; Sosa et al., 2003).
Adding corrosion inhibitors can reduce corrosion
risk. The inhibitors are a small quantity of a compound that controls, reduces
or prevents reactions between a metal and its surroundings (Fouda et al., 2014; Bentiss et al., 2002).
The inhibitor could be a synthetic organic corrosion inhibitor or a green
corrosion inhibitor extracted from natural Resources (Rani and Basu, 2012).
Organic inhibitors, such as thiourea derivatives, are effective in an acid
environment (Shetty and Shetty, 2017). In
seawater, sodium benzoate also has been used as an inhibitor (Nik et al., 2010). However, the
application of synthetic organic corrosion inhibitors is hindered by their
toxicity effect (Popoola, 2019), despite
their high effectivity. On the other side, green organic inhibitors have
recently become an attractive alternative in corrosion prevention research due
to their availability and relatively low cost (Kurniawan,
Mitha, and Fikri, 2018).
The green corrosion
inhibitors are biodegradable and do not contain heavy metals or other toxic
compounds. Extracts of a plant contain a wide variety of organic compounds.
Most of them contain heteroatoms such as P, N, S, and O (Shalabi et al., 2014; Bhawsar, Jain, and Jain, 2013). A
significant number of scientifical studies have been dedicated to the corrosion
inhibition of carbon steel in acidic media by natural products as corrosion
inhibitors (Shalabi et al., 2014; Bhawsar,
Jain, and Jain, 2013; Ramananda, 2013; Majidi et al., 2011; Eddy and Mamza, 2009; Prabhu et al., 2008).
In example, Nicotiana tabacum extract oil has been studied as a corrosion
inhibitor of mild steel in 2 M H2SO4 solution, with an
efficiency of 94.13% at 10 g/L, 303K. Atropa Belladonna extract has also
been investigated on the corrosion of carbon steel in 1 M HCl. It shows 96.6% at 500 ppm using the Tafel
polarization technique. Pradityana et al.
(2017) also used 300 mg/L Myrmecodia Pendans extract as a green
inhibitor of mild steel corrosion in 1 M H2SO4 media with
an efficiency of 64.68%.
Tobacco plants produce more
than 4,000 chemical compounds, including terpenes, alcohols, carboxylic acid,
nitrogen–containing compounds, and alkaloids (Loto
and Popoola, 2011). These components may inhibit electrochemical
activity by chemisorption of the extract components onto the steel surface. It
can reach up to 91.5% on inhibition efficiency, higher than black wattle
(66-87%) and orange peel (80% IE). the chemisorption of tobacco extract
components provides higher effective protection due to the reduction of the
reactivity of metal at the attached sites (Guo et
al., 2018; Shehata, Korshed,
and Attia, 2018). Kurniawan,
Mitha, and Fikri (2018) used tobacco extract as an inhibitor in a 3,5%
NaCl environment with CO2 with excellent results. In this work, we
also use tobacco due to its abundant availability in Indonesia. The H2S
is used as an impurity instead of CO2. The tobacco extract was
obtained from Virginia tobacco leaves using the maceration technique with
demineralized water as solvent. We evaluated the efficiency of tobacco extract
as a green corrosion inhibitor using electrochemical techniques.
2.1. Preparation of tobacco extract
The dried and ground Virginia
tobacco leaves (100 g) were soaked in 100 mL demineralized water for 72 hours
under a room temperature of 27°C. The extract solution was filtered through filter
paper and heated at 50 °C for 3 hours. The extract of tobacco leaf obtained
was used as an inhibitor.
2.2.
Preparation of metal specimens
The material tested was AISI 1018 carbon
steel. Two types of specimens were prepared, i.e. specimens to measure the
corrosion rate (in the form of a solid cylinder with a diameter of 3 mm and a
length of 20 mm) and specimens to determine the inhibition mechanism (in the
form of a 10 mm x 10 mm plate). The carbon steel cylinder is coated with
Teflon®, which acts as an insulator so that only one side of the surface is in
contact with the fluid. The plate specimens are connected by cables and covered
with resin so that only one side is exposed. Before testing, the electrode was
polished using different abrasive papers (up to 2000 grit) and cleaned with
demineralized water and alcohol.
2.3.
Preparation of test media
All chemicals were obtained from Merck,
Germany, without prior treatment. The electrolyte solution is NaCl solution at
a concentration of 35,000 ppm. The solution volume is 50 mL, with and without
adding 600 ppm H2S. Concentrations of tobacco extract are varied at
0, 1000 and 2000 ppm. As in previous research, the H2S gas is made
by reacting Na2S solid with 3 M of HCl solution using Kipp's
apparatus (Rachmawati et al., 2011).
The formed H2S gas is flowed into the NaCl solution for a specific
time to obtain the determined H2S concentration.
2.4.
Corrosion test
Corrosion rate was measured using the
potentiodynamic method and determining the corrosion inhibition mechanism by
using Cyclic Voltammetry (CV), Electrochemical Impedance Spectroscopy (EIS),
X-Ray Diffraction (XRD), and Scanning Electron Microscope (SEM) analyses. The
conventional three-electrode cell is used for the polarization curve, CV and
EIS experiments. Pt-Ir electrode was used as the auxiliary electrode, a
saturated calomel electrode (SCE) was used as the reference electrode, and the
sample was used as the working electrode. All polarization curve tests were
conducted at a scan rate of 5 mV/s. The Gamry Framework and Echem® Analyst
software analyzed the polarization curves. The EIS measurements were performed
with frequencies ranging from 50,000 Hz to 1 Hz, and EIS results were analyzed
using the Gamry Echem® Analyst software. After the corrosion test, the surface
morphologies were observed using SEM. Corrosion phases were detected using XRD
and identified by matching peak positions automatically with Match! Software.
Corrosion rate measurement experiments
were carried out by varying the flow rate, the presence of H2S, and
the concentration of tobacco extracts under room temperature. Then, experiments
to determine the corrosion inhibition mechanism were carried out by varying the
immersion time. The studied carbon steel samples were immersed in 3.5% NaCl
solution with the addition of tobacco extract as an inhibitor for an hour and a
week. The corrosion mechanism was analyzed based on CV and EIS analyses.
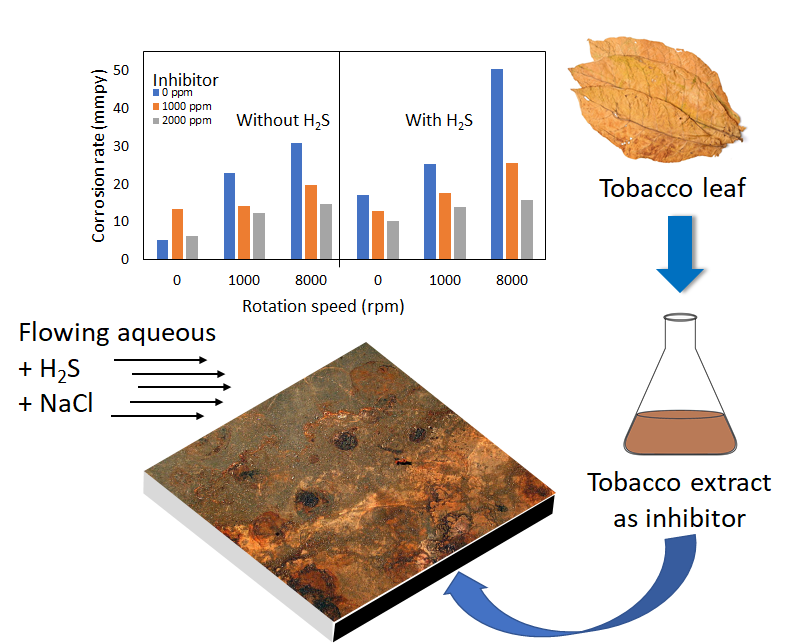
3.1. Corrosion Rate of Carbon Steel in 3.5% NaCl
Solution
The corrosion rate of carbon
steel was determined using the potentiodynamic polarization method against
variations in the flow rate of the solution, the presence of dissolved H2S,
and the concentration of tobacco extract in the electrolyte solution. The
result is shown in Figure 1.
Effect
of H2S - The polarization curve of carbon steel in
NaCl solution with the presence of H2S can be seen in Figure 2a. The
presence of H2S in the 3.5% NaCl solution increased the corrosion
rate. This is due to the dissociation of dissolved H2S into H+
and HS-. The increased concentration of H+ ions in the
solution increases the corrosion rate because the H+ ions can
oxidize carbon steel (Rachmawati et al.,
2011).
Effect
of flow rate - In general, increasing the
flow rate (related to the rotational speed of the electrode) of the solution
will increase the corrosion rate of the carbon steel. The polarization curve in Figure 2b indicates increased dissolved
oxygen as the fluid flow rate increases (Roberge,
2008). The cathodic curve moves to a
higher current with increasing rotational speed.
Effect of tobacco extract - The polarization curve of carbon steel in NaCl solution with the presence of tobacco extract is presented in Figure 3a. The addition of tobacco extract decreased the corrosion rate of the steel in the NaCl solution containing H2S. Adsorption of C-O, N-H, and O-H functional groups from the compounds in the tobacco extract onto the metal surface forms metal protective layers. The presence of these groups in the extract is detected in the Fourier-Transform Infrared Spectroscopy (FTIR) spectrum, as shown in Figure 3b.
Figure 3 (a) Polarization curve of carbon steel in NaCl solution with H2S
and various concentrations of tobacco extract (b) FTIR spectra of tobacco
extract sample
To be noted, the addition of
tobacco extract into NaCl solution in the absence of H2S and under
static conditions increases the corrosion rate. This condition is caused by the
acidity of tobacco extract, which increases the corrosivity of the solution.
The NaCl solution with 1000 and 2000 ppm tobacco extract has pH of 6.4 and 5.9,
consecutively. Formation of a stable, protective layer due to the acid
environment, however, is hindered due to the slow diffusion and adsorption of
tobacco extract's protective compounds onto the steel surface. The addition of
disturbances in the form of flow rate and the presence of H2S
resulted in tobacco extract forming a better protective layer. It inhibits
steel corrosion with an efficiency of 24% to 69% at tobacco extract
concentrations of 1000 ppm and 2000 ppm, respectively.
3.2.
Corrosion Inhibition Mechanism of The Tobacco Extract
Based on the voltammogram in Figure 4,
the corrosion product is unstable since the current ratio at the cathodic peak
to the current at the anodic peak is not equal to 1. The reaction is also
considered irreversible because the potential difference at the anodic and
cathodic peaks exceeds 59 mV. Two cathodic peaks in the voltammogram of samples
in the NaCl solution with H2S and inhibitor solution indicate the
reduction occurred through two reaction stages. Meanwhile, one anodic peak on
the voltammogram indicates that the oxidation reaction occurs in a single
stage.
Figure 4a shows that within the same
potential window and 1-hour immersion, the carbon steels show low corrosion
current, both in a solution of NaCl with 600 ppm of H2S and NaCl with
an inhibitor. The low current indicates a slow corrosion rate of carbon steel.
In Figure 4b, it appears that the anodic current of carbon steel immersed in a
solution of NaCl with 600 ppm of H2S and 2000 ppm of inhibitor for a
week (40 mA) is smaller than the anodic current of steel after immersion for 1
hour (160 mA). This indicates that the tobacco extract, together with the
presence of H2S, can function as an inhibitor. Figure 4b also shows
that within a long exposure time, the corrosivity of H2S is higher
than in tobacco extract.
Testing using EIS is carried out to
determine the electrical circuit equivalent of the ongoing corrosion process.
Three equivalent electrical circuits are generated depending on the type of
electrolyte solution used. The Nyquist plot is shown in Figure 5. Meanwhile,
the equivalent circuit and correlated electrical double layers on the
electrode's surface are presented in Figure 6. Table 1 lists the fitting of the
electrochemical impedance parameters of each configuration.
Figure 6 Equivalent circuit of
steel corrosion in 3.5% NaCl solution and correlated electrical double layers:
(a) in the absence of tobacco extract as an inhibitor, both with and without H2S;
(b) with inhibitor; and (c) with inhibitor and H2S
Electrolyte resistance increased by adding the non-conductive tobacco extract concentration to the electrolyte solution, decreasing electric current and corrosion rate. The addition of tobacco extract and H2S also resulted in increasing in Rct value. The Rct relates to the protective formation layer on the steel surface (Pradityana et al., 2017). A thicker and evenly distributed protective layer inhibits the charge transfer of corrosion reaction (Fouda et al., 2014). The corrosion products of carbon steel in NaCl solution containing H2S and tobacco extract after immersion for one week were analyzed using XRD and can be seen in Figure 7a. Magnetite (Fe3O4) is always present as a corrosion product for the three types of electrolyte solutions used. Immersed in an electrolyte solution of H2S, the steel forms an iron sulfide compound, namely mackinawite (FeS).
Figure 7 (a) X-ray Diffraction
spectra of the corroded carbon steel after one-week immersion in 3.5% NaCl
contains H2S and tobacco extract and morphology of (b) mackinawite
after one-week immersion in 3.5% NaCl solution contains H2S and 2000
ppm of tobacco extract, and (c) magnetite after one-week immersion in 3.5% NaCl
solution contains 2000 ppm of tobacco extract
The existence of mackinawite and magnetite was confirmed by SEM
observations and compared the XRD to the literature, as shown in Figures 7b and
7c.
Tobacco extract can
reduce the corrosion rate of AISI 1018 steel in a flowing NaCl solution containing
H2S, with an inhibition efficiency of 24 – 69% for a concentration
of 1000 – 2000 ppm. The corrosion mechanism of carbon steel in NaCl solution
with and without H2S or tobacco extract starts from the oxidation of
Fe to Fe2+ to form an iron oxide compound in the form of magnetite
(Fe3O4). The presence of H2S causes the
formation of an iron-sulfide compound, namely mackinawite (FeS), as a corrosion
product. Future work will be conducted to study the
operating temperature's effect on tobacco extract inhibitors' stability and
performance.
Al-Janabi, Y.T.,
2020. An Overview of Corrosion in Oil and Gas Industry. In: Corrosion Inhibitors in the Oil and Gas
Industry, Saji V.S., Umoren, S.A., (ed.),
pp. 1–39
Bentiss, F.,
Bouanis, M.M., Marwari, B., Traisnel, M., Lagrenee, M.M., 2002. Effect of
Iodide Ions on Corrosion Inhibition of Mild Steel by
3,5-Bis(4-methylthiophenyl)-4H–1,2,4-Triazole in Sulfuric Acid Solution. Journal
of Applied Electrochemistry, Volume 32,
pp. 671–678
Bhawsar, J.,
Jain, P.K., Jain, P., 2015. Experimental and Computational Studies of Nicotiana
Tabacum Leaves Extract as Green Corrosion Inhibitor for Mild Steel in Acidic
Medium. Alexandria Engineering Journal, Volume 54, pp. 769–775
Dwivedi, D.,
Lepkova, K., Becker, T., 2017. Carbon Steel Corrosion: A Review of Key Surface
Properties and Characterization Methods. RSC Advances, Volume 7, p. 4580
Eddy, N.O.,
Mamza, P.A.P., 2009. Inhibitive and Adsorption Properties of Ethanol Extract of
Seeds and Leaves of Azadirachta Indica on the Corrosion of Mild Steel in H2SO4.
Portugaliae Electrochim. Acta, Volume 27, pp. 443–456
Fouda, A.S.,
Elewady, G.Y., Shalabi, K., Habouba, S., 2014. Tobacco Plant Extracts as Save
Corrosion Inhibitor for Carbon Steel in Hydrochloric Acid Solutions. International
Journal of Advanced Research, Volume 2, pp. 817–832
Gandy, D., 2007.
Carbon Steel Handbook. Electric Power Research Institute, California
Guo, Y., Gao,
M., Wang, H., Liu, Z., 2017. Tobacco Rob Extract as Green Corrosion Inhibitor
for N80 Steel in HCl Solution. International Journal of Electrochemical
Science, Volume 12, pp. 1401–1420
Kahyarian, A.,
Srdjan, N., 2019. H2S Corrosion of Mild Steel: A Quantitative
Analysis of the Mechanism of the Cathodic Reaction. Electrochimica Acta, Volume
297, pp. 676–684
Kim, Y.S., Kim,
J.G., 2017. Corrosion Behavior of Pipeline Carbon Steel under Different Iron
Oxide Deposits in the District Heating System. Metals, Volume 7(5), p.
182
Kurniawan, B.A.,
Mitha, V., Fikri, N., 2018. Effect of Fluid Flow, Ph and Tobacco Extracts
Concentration as Organic Inhibitors to Corrosion Characteristics of AISI 1045
Steel in 3.5% NaCl Environment Containing CO2 Gas. American
Institute of Physics, Volume 1945
Loto, C.A.,
Popoola, A.P.I., 2011. Synergistic Effect of Tobacco and Kola Tree Extracts on
the Corrosion Inhibition of Mild Steel in Acid Chloride. International
Journal of Electrochemical Science, Volume 6, pp. 3264–3276
Majidi, L.,
Znini, M., Cristofari, G., Ansari, A., Bouyanzer, A., Paolini, J., Costa, J.,
Hammouti, B., 2012. Green Approach to Corrosion Inhibition of Mild Steel by
Essential Oil Leaves of Asteriscus Graveolens (Forssk.) in Sulphuric
Acid Medium. International Journal of Electrochemical Science, Volume 7,
pp. 3959–3981
Nik, W.W., Sulaiman, O., Giap, S.E., Rosliza, R.,
2010. Evaluation of Inhibitive Action of Sodium Benzoate on Corrosion Behaviour
of AA6063 in Seawater. International
Journal of Technology, Volume
1(1), pp. 20–28
Popoola, L.T.,
2019. Organic Green Corrosion Inhibitors (OGCIs): A Critical Review. De
Gruyter, Volume 37(2), pp. 71–102
Prabhu, R.A.,
Venkatesha, T.V., Shanbhag, A.V., Kulkarni, G.M., Kalkhamhkar, R.G., 2008.
Inhibition Effects of Some Schiff’s Bases on The Corrosion of Mild Steel in
Hydrochloric Acid Solution. Journal of Corrosion Science, Volume 50, pp. 3356–3362
Pradityana, A.,
Sulistijono, Shahab, A., Noerochim, L., 2017. Sarang Semut (Myrmecodia Pendans)
Extract as a Green Corrosion Inhibitor for Mild Steel in Acid Solution. International Journal of Technology, Volume 1, pp. 48–57
Rachmawati,
I.D., Nurdin, I., Widiatmoko, P., Devianto, H., Irmayanti, F., Saptohadi, S.,
2020. The Effect of Flow Rate and NaCl Concentration on the Corrosion Behavior
of Carbon Steel in NaCl Solutions Containing H2S. IOP Conference Series.: Material Science
and Engineering, Volume 778, p. 012137
Ramananda, M.,
2013. A Green Approach: Corrosion of Mild Steel by Adhatoda Vasica Plant
Extract in 0.5 M H2SO4. Journal of Materials and
Environmental Science, Volume 4, pp. 119–126
Rani, B.E.,
Basu, B.B.J., 2012. Green Inhibitors for Corrosion Protection of Metals and
Alloys: An Overview. International Journal of Corrosion, Volume 2012,
pp. 1–15
Roberge, P.R.,
2008. Corrosion Engineering: Principles and Practice. McGraw-Hill, New
Jersey
Saji, V.S.,
2019a. Research Advancements in Sulfide Scavengers for Oil and Gas Sectors. Reviews
in Chemical Engineering, Volume 37(6),
pp. 663–686
Saji, V.S.,
2019b. Progress in rust converters. Progress in Organic Coating, Volume
127, pp. 88–99
Shalabi, K.,
Abdallah, Y.M., Hassan, H.M., Fouda, A.S., 2014. Adsorption and Corrosion
Inhibition of Atropa Belladonna Extract on Carbon Steel in 1 M HCl Solution. International
Journal of Electrochemical Science, Volume 9, pp. 1468–1487
Shehata, O.S.,
Korshed, L.A., Attia, A., 2018. Green Corrosion Inhibitors, Past, Present,
and Future. Corrosion Inhibitors, principles and Recent
Applications, Volume 6, pp.
121–142
Shetty, S.D.,
Shetty N., 2017. Inhibition of Mild Steel Corrosion in Acid Medium. International Journal of Technology, Volume 5, pp. 909–919
Sosa, E.,
Cabrera, R., Oropeza, M.T., Hernandez, F., Casillas, N., Tremont, R., Cabrera,
C., 2003. Electrochemically Grown Passive Films on Carbon Steel (SAE 1018) in
Alkaline Sour Medium. Electrochim Acta, Volume 48, p. 1665
Wallaert, E.,
Tom, D., Iris, D.G., Verbeken K., 2018. FeS Corrosion Products Formation and
Hydrogen Uptake in a Sour Environment for Quenched & Tempered Steel. Metals,
Volume 8(1), p. 62
Zúñiga, E.M.,
Veloz, M.A., Uruchurtu, J., Reyes, V.E., 2011. Corrosion of Carbon Steel in
Sour Water from the Oil Industry: The Effect of Temperature. International
Journal of Electrochemical Science, Volume 6, pp. 5016–5030
