A Novel Lanthanum-based Solid Oxide Fuel Cell Electrolyte Composite with Enhanced Thermochemical Stability toward Perovskite Cathode
Corresponding email: atiek.noviyanti@unpad.ac.id
Published at : 09 May 2023
Volume : IJtech
Vol 14, No 3 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i3.5189
Noviyanti, A.R., Malik, Y.T., Pratomo, U., Syarif, D.G., 2023. A Novel Lanthanum-based Solid Oxide Fuel Cell Electrolyte Composite with Enhanced Thermochemical Stability toward Perovskite Cathode. International Journal of Technology. Volume 14(3), pp. 669-679
| Atiek Rostika Noviyanti | Department of Chemistry, Faculty of Mathematics and Sciences, Universitas Padjadjaran, Jl. Raya Bandung-Sumedang KM. 21, Jatinangor 45363, Indonesia |
| Yoga Trianzar Malik | Department of Chemistry, Faculty of Mathematics and Sciences, Universitas Padjadjaran, Jl. Raya Bandung-Sumedang KM. 21, Jatinangor 45363, Indonesia |
| Uji Pratomo | Department of Chemistry, Faculty of Mathematics and Sciences, Universitas Padjadjaran, Jl. Raya Bandung-Sumedang KM. 21, Jatinangor 45363, Indonesia |
| Dani Gustaman Syarif | PRTNT-ORTN-BRIN, Jl. Taman Sari 71, Bandung 40132, Indonesia |
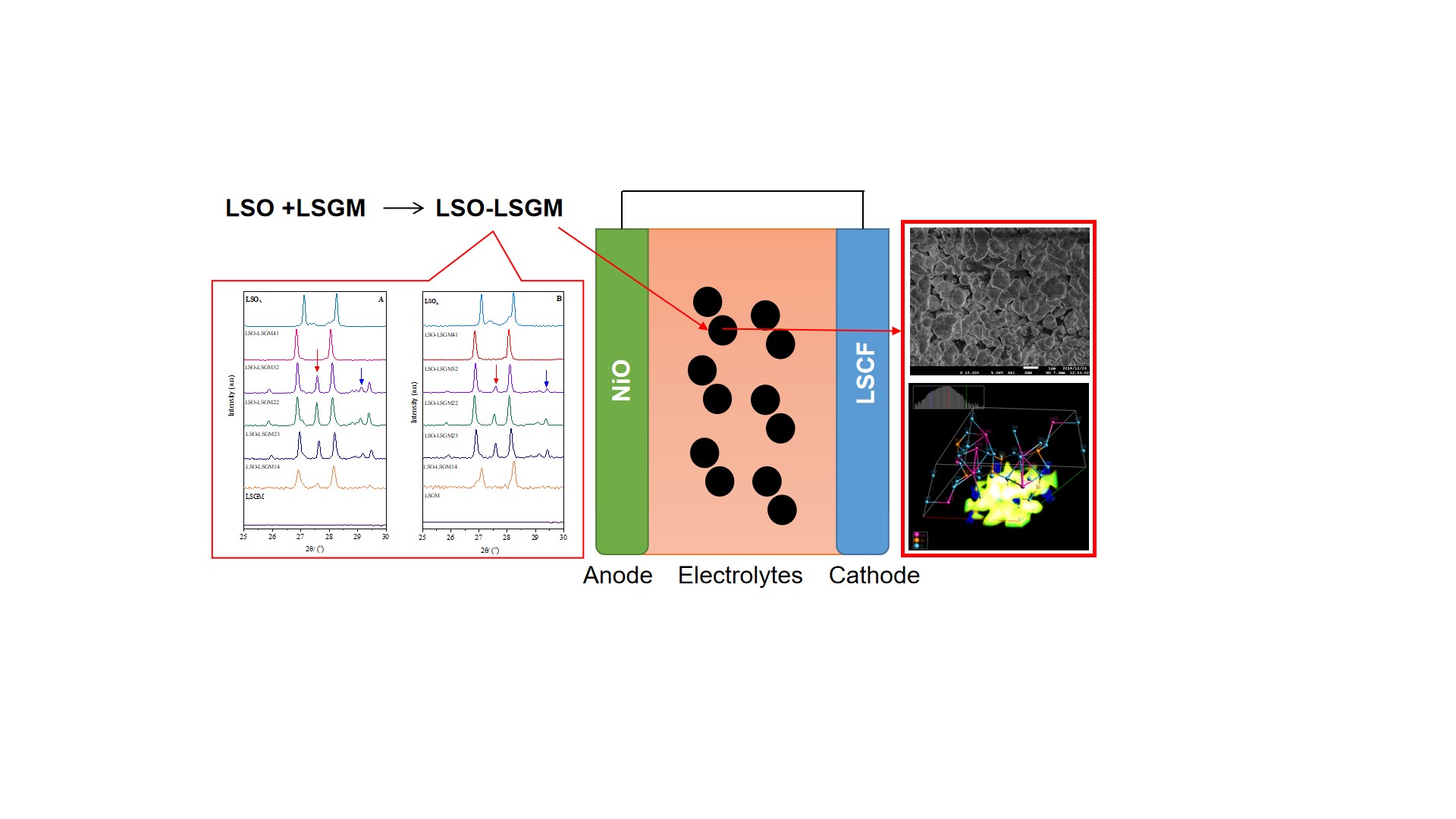
Lanthanum-based electrolytes for Solid Oxide
Fuel Cells (SOFCs) gain extensive attention due to their lower activation
energy and low-cost preparation to convert the energy stored in gaseous
chemicals into electricity. In this context, a La9.33Si6O26-La0.8Sr0.2Ga0.8Mg0.2O2.55
(LSO-LSGM) SOFC electrolyte composite with various mass ratio LSO:LSGM (w/w)
(5:0, 4:1, 3:2, 2:2, 2:3, 1:4) are successfully prepared for the first time
using different LSO precursors with various mass target of 3g (LSO-LSGMA)
and 5g (LSO-LSGMB), respectively. The result shows that the lower
mass target in the synthesis of LSO induced formation of protoenstatite and
coesite secondary phases on the composite of LSO-LSGM based on XRD, FTIR, and
XPS analysis. The SEM micrograph suggests that agglomeration occurred more in
LSO-LSGMA than in LSO-LSGMB. Generally, the composites
signified high chemical stability on La0.8Sr0.2Co0.6Fe0.4O2.55
(LSCF) cathode based on the XRD analysis. The LSO-LSGMA
composites which contained a high percentage of protoenstatite and coesite
resulted in an additional peak of MgSi2Sr, especially for the sample
with the mass ratio of 41 (LSO-LSGM41) suggesting that the chemical stability
of LSO-LSGMA on LSCF cathode is much lower than LSO-LSGMB.
LSCF cathode; LSO-LSGM; Solid oxide fuel cell; Solid state method
The Solid Oxide Fuel Cell (SOFC) is a device that can
generate electricity from gaseous chemicals (hydrogen or short-chained
hydrocarbon). It operates at high temperatures up to 1400K (Abdalla et al., 2018). Similar to the fuel cell (Mulyazmi et
al., 2019), the electrolyte in SOFC portrays an important role
in a set of operating temperatures and thermochemical stability at high
temperatures. Yttrium-stabilized zirconia (YSZ) with general formula (ZrO2)1-x
(Y2O3)x and can generate ionic conductivity
for oxide ion up to 2.00 × 10-1 S.cm-1 (1273K) for an ideal condition (Rahmawati et al., 2017; Preux, Rolle,
and Vannier,
2012). However, a lower
temperature can drop the conductivity to 1.02 × 10-3 S.cm-1
(973K) (Rajesh and Singh, 2014) due to high activation energy (Ea) varied
from 0.89 to 1.16 eV (Zarkov et al., 2015), and another study
noted the value as 1.21 eV (Rajesh and Singh, 2014). YSZ electrolyte is
also reported unavailable to sustain chemical stability with perovskite electrodes such as LSCF and LSM
cathode at 1300 K (Fan, Yan, and Yan, 2011; Minh and Takahashi, 1995).
Lanthanum
silicate oxides (LSO) electrolyte with the formula of La10-x(SiO4)6O2-d,
x=0.1-0.6 has a potential characteristic to substitute YSZ conventional
electrolyte. LSO is more stable than YSZ at high temperatures in the case with
no reactivity to perovskite cathode at 1300 K (Marrero-López, et al., 2010). Moreover, the Ea of LSO is much lower
than YSZ (up to 32 eV) (Kim et al., 2011) despite the
ionic conductivity that is required to be improved. The ionic conductivity of LSO varied from 1.58 × 10-2 to 3.16 × 10-1
S.cm-1 (1273 K) (Higuchi et al., 2010). The structure of LSO is made of an isolated
unit of SiO4 tetrahedral which creates disparate channels
parallel to the c-axis. The smaller of these channels contains lanthanum cations
and vacancies, while the larger one contains both lanthanum cations and oxide
ions which are responsible for the ionic conduction channel (Masson et al., 2017). The lanthanum ions are
coordinated with this isolated tetrahedral SiO4 which can form a
hexagonal structure with P
The composite
strategy is one of the best methods to manage this issue due to its ability to
signify a better result in lowering Ea rather than the doping method.
The composite method also can overcome a reduction issue in some SOFC
electrolytes that may lead to lowering electrical performance (Raza et al., 2020). LSO-YSZ (Noviyanti et al., 2016), LSO-CGO (Noviyanti et al., 2018), and LSGM-YSZ (Raghvendhra et al., 2014) denoted a
decrease of Ea as the addition of the composites, while the addition of Sr-doping to La site in LSO phase
increases the value of Ea two times larger, from 0.56 eV to 1.26 eV (Leon-Reina et al., 2004). LSGM
electrolyte was chosen with LSO for the composite due to the high ionic
conductivity (5.62 × 10-1 S.cm-1 at 1273 K) and a thermal
expansion coefficient (TEC) of 12.7 × 10-6
K-1 (Kharton, Marques,
and Atkinson,
2004), and lower Ea
than YSZ (0.82 eV) (Huang, and Goodenough, 2000). With this
combination of LSO-LSGM, it is expected to result in excellent performance and
high thermochemical stability on the LSCF cathode. Thermochemical stability is
quite important to enhance the electrochemical performance of SOFC cells during
high operating temperatures. The low thermochemical stability will lead to cell
degradation and the short life of SOFC energy generation. However, to obtain a
high-stable electrolyte composite on a typical perovskite SOFC cathode at high
operating temperature, any impurities from constituents of the composite should
be reduced. From all the aforementioned studies, the impurity effect was not
comprehensively studied. Thus, an attempt to design a novel composite of
LSO-LSGM and a thorough study of impurities effect in LSO-LSGM composite on
thermochemical stability of LSO-LSGM on LSCF cathode was carried out to
understand the origin of enhanced thermochemical stability of LSO-LSGM
composite.
To our knowledge, the detailed preparation of LSO-LSGM with various mass ratios of LSO and LSGM and its chemical stability test on the LSCF cathode has not been reported yet. Our study found that the different mass targets during the synthesis of LSO can implicitly induce a formation of protoenstatite and coesite phase in LSO-LSGM which can increase the formation of LaSrGaO4 in the LSO-LSGM/LSCF interface. This study aims to prepare the composites of LSO-LSGM and examines the chemical stability of LSO-LSGM on the LSCF perovskite cathode. The LSO precursor was synthesized using the hydrothermal method accorded with our previous study. In addition, the investigation of the effect of La(OH)3 impurities amount that was found in LSO synthesis on the phase formation in LSO-LSGM composites and chemical stability on the LSCF perovskite cathode was carried out.
2.1. LSO-LSGM preparation
The LSO-LSGM composites were prepared
using the conventional solid-state method (Malik
et
al., 2018).
Generally, the synthesized LSO and commercially-available LSGM were mixed with
the different mass ratios in ethanol using Agate mortar. The mixture was
subsequently dried in the oven at 393K for 2h. The mixture powder was pelleted
with a circular diameter of 15 mm. The pellet of LSO-LSGM was sintered in a
furnace at 1473K for 8h as illustrated in Figure 1. LSOs with 3 and 5 g mass targets were synthesized using
the hydrothermal method as provided in our previous study (Malik et al.,
2019).
LSGM and LSCF were obtained from Sigma (99.99%, metal basis trace). The
composites were divided into two categories: A (using LSO precursors with 3 g
mass target) with various mass ratios of LSO:LSGM (w/w) = 5:0 (LSOA),
4:1 (LSO-LSGMA41), 3:2 (LSO-LSGMA32), 2:2 (LSO-LSGMA22),
2:3 (LSO-LSGMA23), 1:4 (LSO-LSGMA14), 5:0 (LSGM) and B
(using LSO precursor with 5 g mass target) with various mass ratio of LSO:LSGM
(w/w) = 5:0 (LSOB), 4:1 (LSO-LSGMB41),
3:2 (LSO-LSGMB32), 2:2 (LSO-LSGMB22), 2:3 (LSO-LSGMB23),
1:4 (LSO-LSGMB14), 5:0 (LSGM). LSO-LSGM composites were characterized using XRD (D8 Bruker Advanced) at 2range
of 10-70° with Cu K
radiation,
= 0.15418 nm and v = 0.02° min-1 to examine the crystal
structures, Fourier transformation infrared/FTIR (Fischer Scientific) to study
the chemical bonding, X-ray
photoelectron spectroscopy/XPS (JEOL JPS-9010MX) to examine the chemical state
of the composition elements, and SEM (JEOL JSM-7500f) with 10000× magnification
to analyze the microstructure of LSO-LSGM composite electrolytes.
Figure 1 Schematic
illustration of LSO-LSGM electrolyte composite preparation
2.2. Thermochemical stability analysis
Thermochemical stability was assessed
based on the level of LSO-LSGM electrolyte reactivity to the LSCF cathode.
LSO-LSGM electrolytes are mixed with LSCF cathode using ball milling for 2
hours with a mass ratio (w/w) LSO: LSGM of 1:1. This mixture is heated in a
furnace for 48 hours at a temperature of 1473 K. The heating mixture was characterized using XRD Bruker
D8 Advanced, Cu K radiation,
= 0.15418 nm, T = 298 K, v
= 0.02° min-1 in the range of 10-70°. The results of the XRD pattern
are analyzed to observe the possibility of new
crystalline phases emanating from the electrolyte-electrode interactions. The
XRD patterns were refined using Highscoreplus© (Degen et al.,
2014).
3.1. Characterization of Novel LSO-LSGM
Electrolyte Composites
The prepared LSO-LSGM composite was characterized using XRD and FTIR. The results suggested
that the formation of LSO-LSGM was successful. The detail of each
characterization is discussed below.
3.1.1. XRD Characterization
The LSO-LSGM electrolyte composites were
successfully prepared using the solid phase synthesis method. The XRD diffraction patterns varied from each
LSO-LSGM composition (as shown in Figure S1). The main peak intensity of LSGM diffraction at 2= 33° decreases
along with increasing LSO composition in LSO-LSGM composites which are
characterized by
strengthening intensities of LSO peak diffraction. Based on this XRD pattern,
the composting process ran according to our expectations, where both the LSO
and LSGM peaks are found in each LSO-LSGM diffraction composite pattern, in all
LSO-LSGM various mass ratios.
The LSGM deployed in this study has a cubic-based structure with a P m-3m
space group that correlates with La0.8Sr0.2Ga0.8Mg0.2O2.55
(LSGM0802) ICSD No. standard phase. 98-009-8170. The structure of
LSGM with this composition can be maintained in a mixed phase of the LSO-LSGMA32,
LSO-LSGMA22, LSO-LSGMA23, LSO-LSGMA14, and
LSO-LSGMB32, LSO-LSGMB22, LSO-LSGMB23,
LSO-LSGMB14. Meanwhile, for the LSO-LSGMA41 and LSO-LSGMB41
composite electrolytes, the LSGM constituent was compatible with the LSGM phase
of La0.9Sr0.1Ga0.8Mg0.2O2.55
(LSGM0901).
Figure 2 XRD pattern of LSO-LSGMA (left) and LSO-LSGMB
(right) composite electrolytes at the range of 2 = 25-30°. The coesite phase
is indicated at 2
of 27.67° and protoenstantite phase
As shown in Figure 3, the
LSO-LSGM 41 and 14 composites in both categories show a peak phase of LaGaSrO4,
which is a common secondary phase that is often found in LSGM-based
composites. This phenomenon indicates that the LaGaSrO4 phase can be
formed in composites with a low precursor composition concentration of LSGM and
LSO. Moreover, the composition of the LaGaSrO4 phase in the LSO-LSGMA
composite is known to be higher than in the LSO-LSGMB composite
which indicates that the target mass of 5g could be better than LSO with a
target mass of 3g as a precursor of LSO composites. Moreover, the formation of
coesite (SiO2) may closely relate to the preparatory stage of
LSO-LSGM electrolyte composite using the solid-phase method at 1273K (Mart et al., 2008). Meanwhile, the
presence of hydroxide (?OH) species from La(OH)3 impurities in the
LSO precursors contribute to the formation of the protoenstatite (MgSiO3)
phase in LSO-LSGM. This behavior is in line with research by Karakchiev et al. (2009), which explains that a protoenstatite phase can
be formed if species such as Mg2+, OH-, and SiO2
are introduced in the system.
The percentage of impurity phases in the LSO-LSGM electrolyte composite is shown in Table 1. The level of impurities in LSO-LSGMB was much less than that of LSO-LSGMA electrolytes. This behavior indicates that the impurity of La(OH)3 from LSO is assumed to influence the formation of the two phases. Composites prepared from LSO with lower levels of impurities tend to reduce the level of impurities on the composite electrolytes. Thus, the LSO-LSGMB composite electrolyte can be said to have better characteristics compared to the LSO-LSGMA.
Figure 3 XRD pattern of LSO-LSGMA (left) and LSO-LSGMB
(right) composite electrolytes at the range of 2 = 30-33°. LaGaSrO4
phase is indicated at 2? of ~31.5 in LSO-LSGM41 and LSO-LSGM14, respectively.
Table 1 Percentage
of impurity phase in LSO-LSGMA32, LSO-LSGMA22, and
LSO-LSGMA23 electrolyte composites.
|
LSO-LSGMA |
Coesite/ % |
Protoenstatite/ % |
|
LSO-LSGMB |
Coesite/ % |
Protoenstatite/ % |
|
32 |
16.8 |
17.5 |
|
32 |
8.3 |
9.8 |
|
22 |
14.2 |
16.8 |
|
22 |
7.6 |
12.5 |
|
23 |
6.5 |
13.5 |
|
23 |
5.4 |
11.3 |
The composite electrolyte lattice parameter of
LSO-LSGM is shown in Table 2. As shown in Table 2, the LSO phase of the
LSO-LSGM composite has a larger volume of crystal cell units than the standard
LSO (589.26 Å3). Meanwhile, the LSGM phase tends to have a fixed
volume with the standard LSGM (60.01 Å3) except for LSO-LSGMA41.
This result confirmed that the LSO-LSGMA41 correlated to LSGM0901
more than to LSGM0802. The presence of La(OH)3
impurities from high LSO precursors in this composition is thought to affect
the volume expansion behavior of the cell unit of the composite electrolyte.
Another behavior that can be observed from the changes in the volume of
LSO-LSGM electrolyte composite cell units is that it induced an increase in
LSGM cell unit volume which consistently occurred in LSO-LSGM32, LSO-LSGM22,
LSO-LSGM23 (both composite categories) accordingly with the decrease in LSO
cell unit volume (Figure S2). This behavior is closely related to the discovery
of the same type of impurity phase.
Table 2 LSO-LSGMA and LSO-LSGMB
composite electrolyte lattice parameters
|
Category A (LSO-LSGMA) | ||||||||
|
Lattice Parameters | ||||||||
|
|
|
LSOA |
|
|
LSGM | |||
|
LSO-LSGM |
a=b/ Å |
c/ Å |
V/ Å3 |
a=b=c / Å |
V/ Å3 | |||
|
41 |
9.7494(8) |
7.2531(8) |
597.08 |
3.9829(9) |
63.19 | |||
|
32 |
9.7434(6) |
7.2555(0) |
596.53 |
3.9151(9) |
60.01 | |||
|
22 |
9.7372(0) |
7.2527(2) |
595.54 |
3.9154(9) |
60.03 | |||
|
23 |
9.7380(7) |
7.2537(0) |
595.72 |
3.9158(3) |
60.04 | |||
|
14 |
9.6983(4) |
7.1922(4) |
585.87 |
3.9131(8) |
59.92 | |||
|
Category B (LSO-LSGMB) | |||||||||
|
|
|
Lattice Parameters |
| ||||||
|
|
|
LSOB |
|
|
LSGM | ||||
|
LSO-LSGM |
a=b/ Å |
c/ Å |
V/ Å3 |
a/ Å |
V/ Å3 | ||||
|
41 |
9.7465(3) |
7.2517(3) |
596.60 |
3.9171(7) |
60.11 | ||||
|
32 |
9.7435(8) |
7.2552(3) |
596.52 |
3.9131(1) |
59.92 | ||||
|
22 |
9.7400(5) |
7.2538(5) |
595.98 |
3.9139(7) |
59.96 | ||||
|
23 |
9.7365(1) |
7.2536(9) |
595.53 |
3.9148(4) |
60.00 | ||||
|
14 |
9.7198(2) |
7.2411(0) |
592.46 |
3.9133(0) |
59.93 | ||||
3.1.2. FTIR Analysis
To confirm the occurrence of the impurity phases of protoenstantite and
coesite and to investigate the bond interaction in the LSO-LSGM composite, FTIR
analysis was also carried out (see Figure 4). The occurrence of these
impurities may affect the chemical stability of LSO-LSGM with the LSCF cathode.
LSO has the main band at a wave number of 915?980 cm-1 which shows
the SiO4 asymmetric stretch vibration, while LSGM shows the stretch
of Ga-O bonds band at 635 cm-1, La-Mg bonds at
1470 cm-1, and Mg-O bonds at 3368 cm-1 (Byszewski et al., 2006; Baran, 1975). The increasing LSGM mass in the LSO-LSGM
composite affected band changes at 635cm-1 which corresponds to the
number of Ga-O bonds. The vibration band for the -OH bond (3600 cm-1)
that was found in LSO, does not appear at the LSO-LSGM composite. On the other
hand, there is an occurrence of the band from the Mg-O bond shown at 3368 cm-1.
The LSO-LSGMA showed a different peak from LSO-LSGMB at
2800 cm-1, which corresponds to the stretch vibration of Mg-Si of
protoenstatite phases. The Si-O-Si bonds from LSOA considerably have
contributed to an increasing intensity of wave number 1500 cm-1 due
to Mg?Si bonds in the MgSiO3 enstatite phase can occur at
1509 cm-1 (Kalinkina et al., 2001).
3.2. Fourier Map Analysis of LSO-LSGM
The Fourier map analysis was carried out to observe changes in electron
density in the ionic conduction pathway. The result of the Fourier map is shown
in Figure S3 and Figure S4. The green
color represents the high electron density level (positive) in the LSO phase
which becomes more concentrated as the level of LSGM increases. Meanwhile, the
color blue or red represents negative density or low electron density (Masson et al., 2017; Galindo-hernández, and Gómez, 2009).
The different levels
of electron density reinforce the notion that variations in target mass
synthesis can affect the fundamental characteristics of the LSO-LSGM
electrolyte composites. Composite electrolytes containing LSO with higher
impurity levels have lower electron density compared to composite electrolytes
prepared from LSO precursors with lower impurity levels.
3.3. Surface
characterization of LSO-LSGM by Scanning Electron Microscopy (SEM)
Figure 5 SEM micrographs of LSO-LSGMA and LSO-LSGMB (10.000×
magnification)
3.4. X-ray
photoelectron spectroscopy (XPS) of LSO-LSGM
X-Ray
photoelectron spectroscopy was employed to examine the chemical state of the
composition elements and the surface properties of LSO-LSGMA and
LSO-LSGMB. The XPS spectrum is shown in Figure S5. The C1s spectrum displays a
characteristic shoulder due to carbonates at a binding energy (BE) of 289.5 eV.
The strong peak at BE 285.0 eV indicates the presence of amorphous carbon on
the surface. The BE peaks of La
3d, Sr 3d, Ga 2p, and O1s have been observed at 836, 135, 1117, and 530 eV, respectively, which is similar to Raghvendhra et al., (2014). However, Si 2s spectra were not observed at 152 eV for LSO-LSGMA. It may be due to
an overlap with the Ga 3s spectrum that emerged at 153 eV (Kharlamova, 2014).
3.5. Thermochemical stability test of LSO-LSGM
Figure 6 XRD pattern of LSO-LSGMA
(A), LSO-LSGMB
(B) composite
electrolytes at the range of 2 = 27-30°, and (C) Peak ratio of protoenstantite
phase to a neighbour peak of LSO at 2
of 28°
The LSO-LSGM composite has been successfully
prepared. The XRD pattern suggested that LSO-LSGMB has higher
thermochemical stability compared to LSO-LSGMA. This higher
stability originated from the different phase impurities in the LSO precursor
which lead to the formation of protoenstantite and coesite phases in LSO-LSGM
composites. The phases of protoenstatite
and coesite appeared in the LSO-LSGM with the range of mass ratio (LSO to LSGM)
of 3:2 to 2:3. The LSO-LSGMA contained
more coesite of 6.5 to 16.8% and protoenstatite of 13.5 to 17.5% compared to the LSO-LSGMB which showed the coesite phase
of 5.4 to 8.3% and protoenstatite of 9.8 to 11.3%. It is
believed that the amount of LSO precursor affects the formation of
coesite and protoenstatite. The characteristic behavior was confirmed by the
differences in the LSO-LSGM Fourier map which indicates the denser electron
revealed in the LSO structures in the LSO-LSGMB. In
this work, no reactivity of LSO-LSGM occurred on LSCF. The LSO-LSGM
could be the promising SOFC electrolyte with excellent chemical stability
and low impurities through an adjustment of the mass ratio between LSO and
LSGM. Moreover, it can be
confirmed that the mass target could be the
characteristic parameter in the synthesis of LSO. The optimum composition of
LSO-LSGM composite electrolyte with enhanced chemical reactivity toward LSCF
cathode which was found in this study will open a new possibility for the
development of SOFC component with low thermochemical reactivity during the
high operating temperature of SOFC cell through a key parameter of a mass
target during LSO precursor synthesis.
The
authors would like to thank the Academic Leadership Grant 2023 Universitas
Padjadjaran and Directorate General for Higher Education, Ministry of Ristek
Dikti Republik Indonesia (No. 1549/UN6.3.1/PT.00.2023) for the
financial support. The authors also thank the Department of
Applied Chemistry, Tokyo Metropolitan University for allowing the author to use
the XPS instrument.
| Filename | Description |
|---|---|
| R1-CE-5189-20230408165943.pdf | --- |
Abdalla, A.M.,
Hossain, S., Azad, A.T., Petra, P.M.I., Begum, F., Eriksson, S.G., Azad, A.K.,
2018. Nanomaterials for Solid Oxide Fuel Cells: A
Review. Renewable and Sustainable Energy Reviews, Volume 82, pp. 353–368
Adi, W.A., Manaf, A.,
Ridwan, 2017. Absorption Characteristics of the Electromagnetic Wave and
Magnetic Properties of the La0.8Ba0.2FexMn½(1-x)Ti½(1-x)O3
(x = 0.1–0.8) Perovskite System. International Journal of Technology,
Volume 8(5), pp. 887–897
Baran, E.J.,
1975. Infrared Spectrum of LaGaO3. Z.
Naturforsch, Volume 30, pp. 136–137
Byszewski, P.,
Aleksiyko, R., Berkowski, M., Fink-finowicki, J., Diduszko, R., Gebicki, W.,
Baran, J., Antonova, K., 2006. IR
and Raman Spectroscopy Correlation with The Structure of (La/Pr)1-x(Pr/Nd)xGaO3
Solid Solution Crystals. Journal of Molecular Structure, Volume 793, pp. 62–67
Degen, T., Sadki, M., Bron, E., König,
U., Nénert, G., 2014. The HighScore Suite. Powder Diffraction, Volume
29(S2), pp. S13–S18
Fan, B., Yan,
J., Yan, X., 2011. The Ionic Conductivity, Thermal
Expansion Behavior, and Chemical Compatibility of La0.54Sr0.44Co0.2Fe0.8O3- as
SOFC Cathode Material. Solid State Sciences, Volume 13(10), pp, 1835–1839
Galindo-Hernández, F., Gómez, R., 2009.
Fourier Electron Density Maps for Nanostructured TiO2 and TiO2-CeO2 Sol-Gel
Solid. Journal of Nano Research, Volume 5,
pp. 87–94
Higuchi, Y.,
Sugawara, M., Onishi, K., Sakamoto, M., & Nakayama, S. 2010. Oxide Ionic Conductivities of Apatite-Type
Lanthanum Silicates and Germanates and Their Possibilities as an Electrolyte of
Lower Temperature Operating SOFC. Ceramics International, Volume 36(3), pp. 955–959
Huang, K.Q.,
Goodenough, J.B., 2000. A
Solid Oxide Fuel Cell Based on Sr- And Mg-Doped LaGaO3 Electrolyte:
The Role of a Rare-Earth Oxide Buffer. Journal of Alloys and Compounds. Volume 303, pp. 454–464
Kalinkina, E.V,
Kalinkin, A.M., Forsling, W., Makarov, V.N., 2001. Sorption of atmospheric carbon dioxide and structural
changes of Ca and Mg silicate minerals during grinding. International
Journal of Mineral Processing.
Volume 61(4), pp. 289–299
Karakchiev,
L.G., Avvakumov, E.G., Gusev, A.A., Vinokurova, O.B., 2009. Possibilities of Exchange Mechanochemical
Reactions in the Synthesis of Magnesium Silicates. Chemistry for Sustainable Development, Volume 17, pp. 581–586
Kharlamova,
T.S., Matveev, A.S., Ishchenko, A. V, Salanov, A.N., Koshcheev, S. V, Boronin,
A.I., Sadykov, V.A., 2014. Synthesis and Physicochemical and Catalytic
Properties of Apatite Type Lanthanum Silicates. Kinetics and
Catalysis, Volume 55(3), pp. 361–362
Kharton, V.V.,
Marques, F.M.B., Atkinson, A., 2004.
Transport Properties of Solid Oxide Electrolyte Ceramics: A Brief Review. Solid
State Ionics, Volume 174(1–4), pp.
135–149
Kim, Y., Shin,
D.-K., Shin, E.-C., Seo, H.-H., Lee, J.-S., 2011. Oxide Ion Conduction Anisotropy Deconvoluted in
Polycrystalline Apatite-Type Lanthanum Silicates. Journal of Materials
Chemistry, Volume 21, pp. 2940–2949
León-Reina, L.,
Losilla, E.R., Martínez-Lara, M., Bruque, S., Aranda, M.A., 2004. Interstitial
Oxygen Conduction in Lanthanum Oxy-Apatite Electrolytes. Journal of
Materials Chemistry, Volume 14(7), pp. 1142–1149
Malik, Y.T.,
Noviyanti, A.R., Syarif, D.G., 2018.
Lowered Sintering Temperature on Synthesis of La9.33Si6O26
(LSO) – La0.8Sr0.2Ga0.8Mg0.2O2.55
(LSGM) Electrolyte Composite and the Electrical Performance on La0.7Ca0.3MnO3
(LCM) Cathode. Journal of Scientific and Applied Chemistry, Volume
21(4), pp. 205–2010
Martinez, J.R.,
Vázquez-Durán, A., Martinez-Duran, G., Ortega-Zarzosa, G., Palomares-Sánchez,
S.A., Ruiz, F., 2008. Coesite Formation at Ambient
Pressure and Low Temperatures, Advances in Materials Science and
Engineering, Volume 2008,
p. 406067
Masson, O.,
Berghout, A., Béchade, E., Jouin, J., Thomas, P., Asaka, T., Fukuda, K., 2017. Local Structure and Oxide-Ion Conduction
Mechanism in Apatite-Type Lanthanum Silicates. Science and Technology of
Advanced Materials, Volume 18(1), pp. 644–653
Minh, N.Q.,
Takahashi, T., 1995. Cathode. Science and Technology
of Ceramic Fuel Cells. Amsterdam: Elsevier Science B.V.
Mulyazmi, Daud, W.R.W., Rahman, E.D.,
Purwantika, P., Mulya, P.A., Sari, N.G., 2019. Effect of Operating Conditions
on the Liquid Water Content Flowing Out of the Cathode Side and the Stability
of PEM Fuel Cell Performance. International Journal of Technology, Volume 10(3),
pp. 634–643
Noviyanti, A.R.,
Hastiawan, I., Eddy, D.R., Adham, M.B., Hardian, A., Syarif, D.G. 2018. Preparation and Conductivity Studies of La9.33Si6O26
(LSO) -CeE0.85Gd0.15O1.925 (CGO15)
Composite Based Electrolyte for IT-SOFC. Oriental Journal of Chemistry,
Volume 34(4), pp. 2125–2130
Noviyanti, A.R.,
Irwansyah, F.S., Hidayat, S., Hardian, A., Syarif, D.G., Yuliyati, Y.B.,
Hastiawan, I., 2016. Preparation and Conductivity of
Composite Apatite La9.33Si6O26 (LSO)-Zr0.85Y0.15O1.925
(YSZ). In: AIP Conference Proceedings, Volume 1712(1), p. 050002
Noviyanti,
A.R., Juliandri, J., Winarsih,
S., Syarif D.S., Malik, Y.T., Septawendar,
R., Risdiana R., 2021. Highly Enhanced Electrical Properties of
Lanthanum-Silicate-Oxide-Based SOFC Electrolytes with Co-Doped Tin and Bismuth
in La9.33-xBixSi6-ySnyO26.
RSC Advances, Volume 11, pp. 38589–38595
Preux, N., Rolle, A., Vannier, R.N., 2012. Electrolytes and Ion Conductors Forfor Solid Oxide Fuel
Cells (SOFCs). Woodhead
Publishing Limited
Raghvendra,
Singh, R.K., Singh, P., 2014.
Electrical Conductivity of LSGM-YSZ Composite Materials Synthesized via Coprecipitation
Route. Journal of Materials Science, Volume 49(16), pp. 5571–5578
Rahmawati, F., Permadani, I., Syarif, D.,
Soepriyanto, S., 2017. Electrical Properties of Various Composition of Yttrium
Doped-Zirconia Prepared from Local Zircon Sand. International Journal of
Technology, Volume 8(5), pp. 939–946
Rajesh, R.,
Singh, K., 2014. Electrical Conductivity of LSGM –
YSZ Composite Materials Synthesized via Coprecipitation Route. Journal of Materials Science, Volume 49(16), pp. 5571–5578
Raza, R., Zhu,
B., Ra, A., Raza, M., Lund, P., 2020.
Functional Ceria-Based Nanocomposites for Advanced Low- Temperature (300-600°C) Solid Oxide Fuel Cell: A Comprehensive
Review. Materials Today Energy, Volume 15, p. 100373
Zarkov, A., Stanulis, A., Sakaliuniene, J., Butkute, S., 2015. On The Synthesis of Yttria-Stabilized
Zirconia: A Comparative Study. Journal of Sol-Gel Science and Technology.
Volume 76(2), pp. 309–319
