Biosorption of Hexavalent Chromium Cr(VI) using Microalgae Scenedesmus sp as Environmental Bioindicator
Corresponding email: rudi_biokimia@yahoo.com
Published at : 28 Jun 2023
Volume : IJtech
Vol 14, No 4 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i4.5188
Kartika, R., Ritonga, A.H., Sulastri, L., Nurliana, S., Irawan, D., Simanjuntak, P., 2023. Biosorption of Hexavalent Chromium Cr(VI) using Microalgae Scenedesmus sp as Environmental Bioindicator. International Journal of Technology. Volume 14(4), pp. 791-799
| Rudi Kartika | Faculty of Mathematics and Natural Science, Mulawarman University, Samarinda-75119, Indonesia |
| Ahmad Hafizullah Ritonga | Institut Kesehatan Medistra Lubuk Pakam, Deli Serdang-20512, Indonesia |
| Lilik Sulastri | Sekolah Tinggi Teknologi Industri dan Farmasi, Bogor-16151, Indonesia |
| Siti Nurliana | Balai Riset dan Standarisasi Industri Samarinda, Samarinda-75124, Indonesia |
| Deddy Irawan | Departement of Chemical Engineering, Politeknik Negeri Samarinda, Samarinda-75131, Indonesia |
| Partomuan Simanjuntak | Research Center for Pharmaceutical Ingredient and Traditional Medicine, National Research and Innovation Agency (BRIN), KST Soekarno Cibinong 16911, Indonesia |
Scenedesmus
sp. is a freshwater green alga that functions as an ionic biosorbent and can
also be a bioindicator for water contaminated with hexavalent chromium Cr(VI)
ion. This study aimed to observe the growth of Scenedesmus sp. exposed to Cr(VI) ion at various concentrations and
analyze the remaining Cr(VI) ion that did not undergo biosorption by
microalgae. This research was conducted on Scenedesmus
sp. microalgae growth media using five bioreactors, each with a different
Cr(VI) ion exposure concentration. The remaining ion in the growth media was
analyzed for its concentration with an ultraviolet-visible spectrophotometer at
time variations with an interval of two days. Maximum biosorption with exposure
to Cr(VI) occurred at a concentration of 1.0 ppm on day 12 of 99.93%. At
concentrations of 5.0 ppm and 7.0 ppm, microalgae growth was very poor,
indicating the medium was toxic.
Biosorption; Hexavalent Chromium; Scenedesmus sp; Toxicity
The microalga Scenedesmus sp. is highly competent at
binding inorganic ions such as carboxyl, amine, sulfate, and sulfonate, which
lends itself viable to treat aquatic waste. Microalgae have the advantage of
being environmentally friendly, recyclable, and low maintenance costs (Wilan et al., 2020). Scenedesmus
sp. is a cosmopolitan microalga that lives in colonies within brackish water
and soil with a humid climate. Their cells are cylindrical (8-20 m in length
and 3-9 m in width) and are surrounded by three layers consisting of an inner
layer (cellulose), a middle layer (membrane structure), and an outer layer net
of pectin and fine hairs (Prihantini,
Damayanti, and Yuniati, 2007).
Scenedesmus sp. is widely utilized as a supplement, fish feed, pollutant
removal agent for wastewater treatment, a source of biofuel, and a
bio-indicator of water pollution using herbicides as a determinant (Fodorpataki, Bartha, and
Keresztes, 2009; Makareviciene et al., 2011; Sudibandriyo and Putri,
2020).
Industrial activities often
pollute their surrounding with various classes of contaminants, of which heavy
metals are particularly concerning since they persist in the environment and do
not decompose or degrade into benign compounds as most organic pollutants do.
Heavy metal ions are toxic to aquatic ecosystems and human health above a
certain concentration level (Suprapto
et al., 2020).
Heavy metal ions can be
removed from water through several methods, such as physical adsorption,
chemical sedimentation, mechanical filtration, and ion exchange. However, these
processes have their drawbacks, such as secondary pollution due to the chemicals
used and high cost. An environmentally friendly alternative is using
microorganisms to adsorb the ions out of the water, a technique known as
biosorption. This method is highly efficient in wastewater detoxification, and
it has a simple implementation and a low cost. Microorganisms' adsorption of
heavy metal ions is a rapid and reversible process in which the cell wall
serves as a binding site, which means that the microorganism does not even need
to be alive for this purpose. Using dead microbial cells could be more
cost-efficient because they do not require a supply of nutrients during the
process. Several factors affect biosorption: characteristics of biomass,
temperature, pH, biosorbent concentration, contact time, and biomass surface
area. The biomass must be immobilized to avoid blockage of the reaction (Wilan et al., 2020).
Many techniques have been
applied to improve the performance of a biosorbent. The chemical composition of
the adsorbing surface may be modified by adding or removing certain functional
groups to improve specificity and binding energy. The binding surface area may
be expanded by increasing porosity (Anuar et al., 2019). Several
researchers have used the biosorption method to remove heavy metals in solution
using dead biomass to bind pollutants through simultaneous adsorption, complex
formation, micro-surface deposition, and ion exchange (Kusrini et al.,
2019; Fomina and Gadd, 2014; Ekmekyapar et al., 2012). Certain bacteria
can absorb Pb ions, such as micrococcus sp. and flavobacterium sp., by up to
100% at an initial concentration varying from 2.0 ppm to 10 ppm after an
exposure of 3 to 30 days (Susanto,
Kartika, and Koesnarpadi, 2019).
Chromium is a very toxic
and dangerous heavy metal. Among the valence range of chromium from -2 to +6,
only hexavalent chromium (Cr VI) and trivalent chromium (Cr III) have
environmental significance due to their stability in the form of oxidation in
water and poor absorption by soil and organic matter, making them slow to
sediment out of the solution (Mnif et al., 2017).
Cr (VI) compounds are
generated by various industries such as metallurgy, leather tanning, paint,
textile, pulp, ore and petroleum refining, metal corrosion, and electroplating.
Those compounds may be released into the environment due to leakage, poor storage,
or improper disposal. Chromium ions are toxic in the human body because they
can irritate the respiratory tract, blood vessels, kidneys, and skin at high
levels. According to the World Health Organization (WHO) drinking water
guidelines, the maximum recommended limit for total chromium is 0.05 ppm (Rahman
and Singh, 2019; Khatoon and Rai, 2016; Khatoon et al., 2013).
This study aims to observe
the growth of Scenedesmus sp. exposed
to Cr(VI) ion at various concentrations in the growth medium, during which the
alga should adsorb the ions, and then analyze the remaining Cr(VI) ion in the
growth medium at an interval of two days. The extent of absorption of Cr(VI)
ion can be a bioindicator for the environment by providing information about
the growth of the microalgae Scenedesmus
sp., which is disturbed at a certain concentration and is characterized by
a colorless growth media (not growing or dying). However, if the growth medium
is green, the growth is normal (not disturbed by Cr(VI) ion).
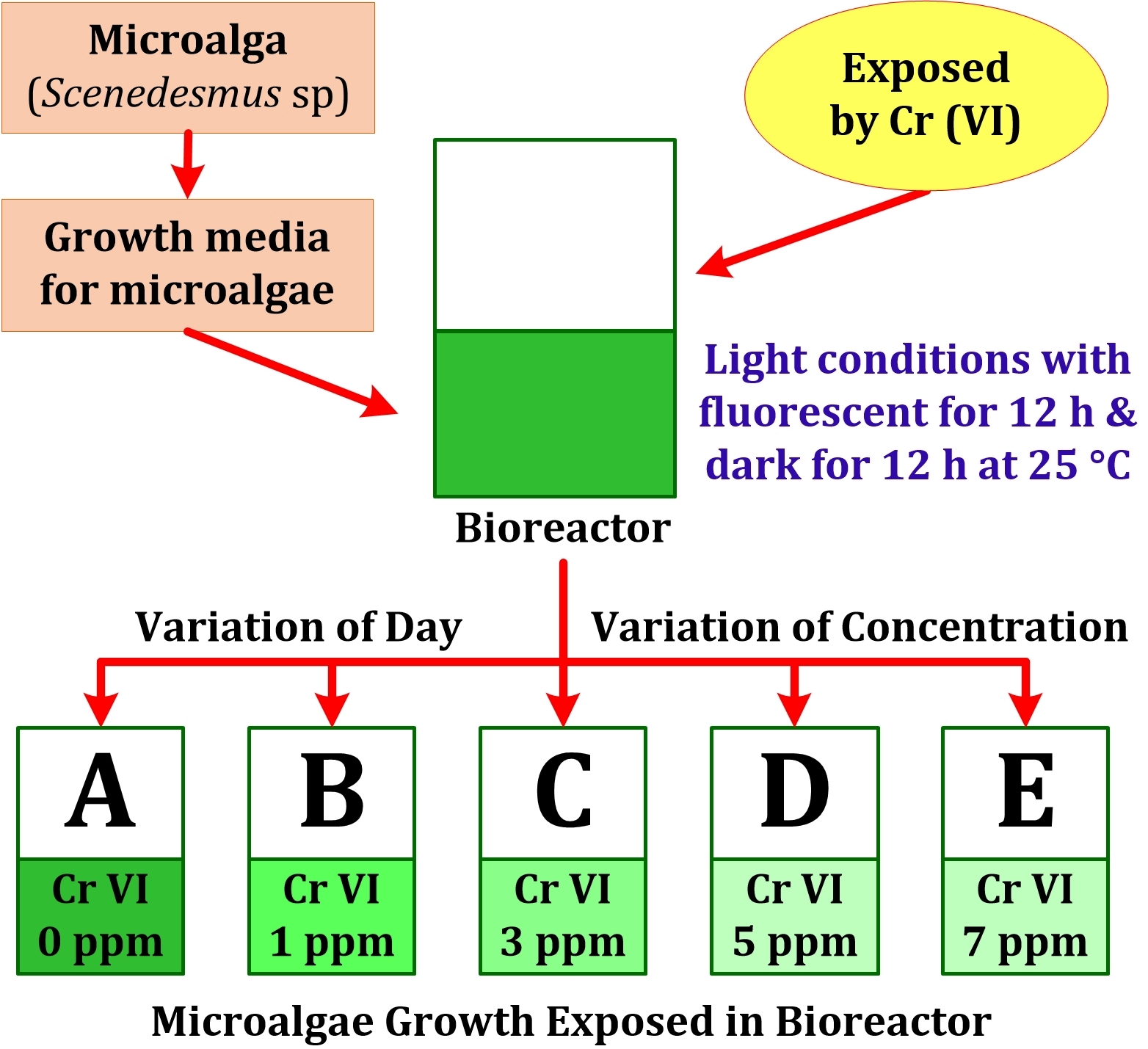
Figure 1 Scheme of (a)
Microalgae Cultivation, (b) Exposed to
Bioreactors
2.2. Preparation of Cr(VI) Standard Solution
A mass of K2Cr2O7
weighing 0.1414 g was dried in an oven and dissolved in 100 mL distilled water
in a volumetric flask to yield a Cr(VI) 500 ppm solution. 10 mL of the Cr(VI)
500 ppm solution was diluted with 100 mL distilled water in a volumetric flask
to obtain a Cr(VI) 50 ppm solution. 10 mL of Cr(VI) 50 ppm solution was diluted
with 100 mL distilled water in a volumetric flask to obtain a standard Cr(VI) 5
ppm solution.
2.3. Curve
Calibration
2 mL of the Cr(VI) 5 ppm standard solution was added into a
100 mL volumetric flask, followed by five drops of H3PO4.
The pH of the mixture was adjusted by adding 0.2 M H2SO4
until it reached pH 2. Next, 2 mL of diphenylcarbazide was added, and the flask
was filled with distilled water up to the marked line, resulting in a 0.1 ppm
standard solution for the calibration curve. The procedure was repeated with
the volume of the Cr(VI) 5 ppm standard solution incremented by 2 mL up to 20 mL,
resulting in standard solutions with a concentration of 0.2 ppm, 0.3 ppm, 0.4
ppm, 0.5 ppm, 0.6 ppm, 0.7 ppm, 0.8 ppm, 0.9 ppm, and 1.0 ppm. The solutions
were each rested for 10 min before their absorbances were measured at a
wavelength of 540 nm.
2.4. Measurement of Chromium Concentration
A
10 mL sample of the culture solution was filtered using a folder membrane at
0.45 microns. It was treated according to a calibration curve standard
solution, and the concentration was measured at a wavelength of 540 nm.
2.5. Determination
of Remaining Cr(VI) Ion Concentration in Growth Medium with Time Variations
The concentration of Cr(VI) ion in the culture medium was
measured by taking a 10 mL sample and running it through a vacuum filter using
a millipore membrane (0.4 microns), then determining the concentration of
Cr(VI) ion. The measurement was performed on the initial solution, then every
other day up to the twelfth day. The Cr(VI) ion which has undergone biosorption
is the concentration of Cr(VI) ion obtained (ppm) reduced with the
concentration of Cr(VI) ion remaining in the medium.
Table 1 shows that the Cr(VI) ion concentration decreased with increasing contact time. The longer the exposure time, the larger the possible interactions between the biosorbent material and the metal ions, which allowed more active groups to bind metal ions and increase the number of metal ions absorbed. The biosorption proceeded with increasing contact time until the equilibrium point was reached. The length of contact time affected the metal ion-binding process by the biosorbent surface before the surface reached the saturation point. When the biosorbent has reached the equilibrium point, the biosorbent will not bind any heavier metals because the surface of the cell wall is saturated.
Table
1 Absorption of Cr(VI) with
variations in concentration and time
Based on the
concentration of Cr(VI) ion exposed and remaining in the growth medium, the
percentage of biosorption can be determined based on the following equation (Vendruscolo,
da Rocha Ferreira, and Antoniosi Filho, 2017):
Note Ce = Concentration of Cr(VI) ion
exposed in the growth medium (ppm)
Cr = Concentration of
Cr(VI) ion remaining in the growth medium (ppm)
Based on Table 1 and the percentage of
biosorption equation, the calculation results for the percentage of Cr(VI) ion
removal are listed in Table 2 below.
Table 2 Percentage of Cr(VI) ion removal with
variations in concentration and time
|
Cr(VI) ions removal (%) | ||||
|
Day |
1 ppm |
3 ppm |
5 ppm |
7 ppm |
|
0 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
|
2 |
14.7766 |
7.1836 |
2.3320 |
2.6836 |
|
4 |
26.4605 |
13.5690 |
3.6351 |
4.6139 |
|
6 |
35.5670 |
18.2440 |
4.4582 |
5.9322 |
|
8 |
57.6632 |
33.2953 |
6.6968 |
7.7067 |
|
10 |
76.4261 |
44.2987 |
7.4273 |
7.6733 |
|
12 |
99.9313 |
51.4253 |
10.2030 |
8.0410 |
Figure 2 (a) Plots of Cr(VI) concentration as a function of time, (b) plots of the
percentage of Cr(VI) removal as a function of time, and (c) the plots of the
percentage of Cr(VI) removal as a function of Cr(VI) concentration
At Cr(VI)
3.0 ppm, the a similar trend of increasing ion absorption throughout the study
period. However, the amount of chromium ion absorbed was lower than the Cr(VI)
1.0 ppm exposure, which meant that Cr(VI) ion was still absorbed but was toxic.
The growth of Scenedesmus sp. was
disrupted when exposed to this level of chromium because the metal ion cofactor
required by its enzymes was non-competitively inhibited, and the complex
reagents exchange metal ions from the enzyme exceeded their tolerance limit (Daneshvar et al.,
2019; Susanto,
Kartika, and Koesnarpadi, 2019).
At Cr(VI) 5.0 ppm, the absorption of
Cr(VI) dropped precipitously, indicating that the solution was already highly
toxic to the microalga and no microbial growth was occurring. The same result
was obtained from the 7.0 ppm medium, and in both media, no green color
developed beyond the initial very pale green color. It is a bio-indication that
the growth media already contained chromium ions at high concentrations (Susanto,
Kartika, and Koesnarpadi, 2019).
The reduction of ion
concentration in the growth media was due to (1) the biosorption with bonds
between metallothionein thiol groups, namely polypeptides containing about 30%
of the amino acid cysteine (Dewi, Yuniastuti, and Ahmed, 2018), and (2) the non-competitive inhibitory effect of
Cr(VI) ion to form mercaptide salts with sulfhydryl groups of enzyme proteins :
Notes: M = Metal, R = Protein radicals from microalgae, and SH = Sulfhydryl
This condition
inhibits the action of the enzyme because it is not similar to the cofactor as
an activator of the enzyme (Dewi, Yuniastuti, and Ahmed, 2018).
Figure 3 Microalgae growth exposed to Cr(VI) bioreactors of 0; 1; 3; 5, and 7 ppm
The growth medium without any Cr(VI)
(reactor A) did not manifest the presence of Cr(VI), and the growth of
microalgae was vigorous, as shown in Figure 3, in which the 0.0
ppm medium was deep green. Meanwhile, in the growth media contaminated with
Cr(VI) 1.0 ppm (reactor B), the absorption process occurred from day second to
twelfth, and the concentration of remaining ions in the growth medium was
reduced to 0 ppm on day twelfth (99.93% absorbed). It showed good absorption at
exposure to a concentration of 1.0 ppm, and only the growth was slightly
disturbed.
The medium exposed to Cr(VI)
3.0 ppm (reactor C) had its Cr(VI) concentration reduced by 50% after twelve
days of incubation. The medium exposed to Cr(VI) at 5.0 ppm (reactor D) had its
Cr(VI) concentration reduced by only about 10.29% in the growth medium after
twelve days. Likewise, the growth medium exposed to Cr(VI) at 7.0 ppm (reactor
E) had its Cr(VI) concentration reduced by about 8.05% in the growth medium,
which indicated poor growth in reactor D and reactor E. Both of these reactors
have the potential to be toxic to microalga growth. This is the result of a
comparison with several organisms used as bio-sorbents and the mechanism that
occurs in the absorption of Cr(VI) ions stated in Table 3.
Table 3 Several types of biosorbents and mechanism of
Cr(VI) ion removal
|
Name of Organism |
Isolation Site |
Mechanism of Cr Removal |
Initial Cr (VI) Concentration (mg/L) |
Remediation (%) |
|
Acinetobacter
junii |
Chromite mine site |
Reduction |
54 |
99.95 |
|
Cellulosimicro-bium
funkei strain AR6 |
Leather industry effluent contaminated soil |
Biosorption, Reduction |
250
|
80.43
|
|
Pseudomonas
stutzeri L1 |
Crude oil |
Biosorption, Reduction |
100-1000
|
97
|
|
Acinetobacter
baumannii L2 |
Crude oil |
Biosorption, Reduction |
1000 |
99.58
|
|
Pleurotus
ostreatus |
Mushroom farms |
Biosorption |
500 |
80 |
|
Acremonium sp.
|
Tannery effluent contaminated soil |
Biosorption |
100 |
90 |
|
Penicillium
griseofulvum MSR1 |
Tannery effluent |
Biosorption |
67.8 |
79.9 |
|
A. niger |
Contaminated soil |
Biosorption |
125 |
96.3 |
|
Saccharomyces
cerevisiae |
Culture collection bank |
Biosorption |
200 |
85 |
|
Opuntia
cladodes |
Aqueous solution |
Biosorption |
18.5 |
83 |
Source : (Jobby et
al., 2018; Fernandez-Lopez, Angosto,
and Aviles, 2014)
The results of this research can pave the way for a novel bioindicator
device to be used by premises that produce a waste stream containing Cr(VI)
ions. The growth color, which shows a paler color (slowest growth), indicated
high Cr(VI) waste. The wastewater treatment system that would process the
stream containing Cr(VI) generated by an industrial activity can be augmented
with a pond overgrown with Scenedesmus sp. microalgae. If the growth of
Scenedesmus sp. microalgae is vigorous, exhibiting a deep green color in the
water, then the waste quality is suitable for discharge. Otherwise, if the
growth of Scenedesmus sp. microalgae is inhibited, exhibiting a pale green
color or no color, then the water needs more treatment before discharge.
The
microalga absorbed Cr(VI) well (99.93%) after twelve days of incubation in a
medium containing 1.0 ppm chromium. Incubating for twelve days in a medium with
3.0 ppm chromium resulted in only 50% absorption. The mediums with 5.0 ppm and
7.0 ppm chromium were toxic to the microalga, with very little chromium
absorbed. This technique may be utilized as an environmental bioindicator for
companies that generate Cr(VI) ion waste in their process to test their
wastewater before discharging it into water bodies or the environment.
Anuar, F.I., Hadibarata, T., Muryanto,
Yuniarto, A., Priyandoko, D., Sari, A.A., 2019. Innovative Chemically Modified
Biosorbent for Removal of Procion Red. International Journal of Technology,
Volume 10(4), pp. 291–319
Daneshvar, E., Zarrinmehr, M.J., Kousha, M.,
Hashtjin, A.M., Saratale, G.D., Maiti, A., Vithanage, M., Bhatnagar, A., 2019.
Hexavalent Chromium Removal from Water by Microalgal-Based Materials:
Adsorption, Desorption and Recovery Studies. Bioresource technology,.
Elsevier Volume 293pp. 122064
Dewi, N.K., Yuniastuti, A., Ahmed, A.M.A.,
2018. Identification of Metallothionein Gene in Human Plasma: A Molecular
Analysis of Cadmium and Lead Pollution in Gas Station Environment. Jurnal
Pendidikan IPA Indonesia, Volume 7(4), pp. 383–390
Ekmekyapar, F., Aslan, A., Bayhan, Y.K.,
Cakici, A., 2012. Biosorption of Pb (II) by Nonliving Lichen Biomass of
Cladonia Rangiformis Hoffm. International Journal of Environmental Research, International Journal of Environmental Research (IJER) Volume 6(2), pp. 417–424
Fernandez-Lopez, J.A., Angosto, J.M., Aviles,
M.D., 2014. Biosorption of Hexavalent Chromium from Aqueous Medium with Opuntia
Biomass. The Scientific World Journal, Hindawi Volume 2014
Fodorpataki, L., Bartha, C., Keresztes, Z.G.,
2009. Stress-Physiological Reactions of the Green Alga Scenedesmus Opoliensis
to Water Pollution with Herbicides. Aquatic, Volume 17(1), pp. 51–56
Fomina, M., Gadd, G.M., 2014. Biosorption:
Current Perspectives on Concept, Definition and Application. Bioresource Technology,
Volume 160, pp. 3–14
Jobby, R., Jha, P., Yadav, A.K., Desai, N.,
2018. Biosorption and Biotransformation of Hexavalent Chromium [Cr(VI)]: A
Comprehensive Review. Chemosphere, Elsevier Ltd Volume 207, pp. 255–266
Khatoon, H., Rai, J.P.N., 2016. Agricultural Waste
Materials as Biosorbents for the Removal of Heavy Metals and Synthetic Dyes-a
Review. Octa Journal of Environmental Research, Scientific Planet
Society Volume 4(3), pp. 208–229
Khatoon, N., Khan, A.H., Pathak, V.,
Agnihotri, N., Rehman, M., 2013. Removal of Hexavalent Chromium from Synthetic
Waste Water Using Synthetic Nano Zero Valent Iron (NZVI) as Adsorbent. International
Journal of Innovative Research in Science, Engineering and Technology,
Volume 2(11), pp. 2319–8753
Kusrini, E., Wu, S., Susanto, B.H., Lukita,
M., Gozan, M., Hans, M.D., Rahman, A., Degirmenci, V., Usman, A., 2019.
Simultaneous Absorption and Adsorption Processes for Biogas Purification Using
Ca (OH) 2 Solution and Activated Clinoptilolite Zeolite/Chitosan Composites. International
Journal of Technology, Faculty of Engineering Universitas Indonesia Volume
10(6), pp. 1243–1250
Makareviciene, V., Andruleviciute, V.,
Skorupskaite, V., Kasperoviciene, J., 2011. Cultivation of Microalgae Chlorella
Sp. and Scenedesmus Sp. as a Potentional Biofuel Feedstock. Environmental
Research, Engineering and Management, Volume 57(3), pp. 21–27
Mnif, A., Bejaoui, I., Mouelhi, M., Hamrouni,
B., 2017. Hexavalent Chromium Removal from Model Water and Car Shock Absorber
Factory Effluent by Nanofiltration and Reverse Osmosis Membrane. International
journal of analytical chemistry, Hindawi Volume 2017
Prihantini, N.B., Damayanti, D., Yuniati, R.,
2007. The Effect of Tauge Extract Medium (TEM) Concentration to the Growth of
Subang Isolated Scenedesmus. Makara Journal of Science, Univ Indonesia
Directorate Research & Public Serv, UI Campus. Volume 11(1), pp. 1–9
Rahman, Z., Singh, V.P., 2019. The Relative
Impact of Toxic Heavy Metals (THMs)(Arsenic (As), Cadmium (Cd), Chromium
(Cr)(VI), Mercury (Hg), and Lead (Pb)) on the Total Environment: An Overview. Environmental
monitoring and assessment, Springer Volume 191(7), pp. 1–21
Sudibandriyo, M., Putri, F.A., 2020. The
Effect of Various Zeolites as an Adsorbent for Bioethanol Purification Using a
Fixed Bed Adsorption Column. International Journal of Technology, Faculty of Engineering Universitas Indonesia Volume 11(7), pp. 1300–1308
Suprapto, Gotoh, T., Humaidah, N.,
Febryanita, R., Firdaus, M.S., Ningrum, E.O., 2020. The Effect of Synthesis
Condition of the Ability of Swelling, Adsorption, and Desorption of
Zwitterionic Sulfobetaine-Based Gel. International Journal of Technology,
Volume 11(2), pp. 291–319
Susanto, A., Kartika, R., Koesnarpadi, S.,
2019. Lead Biosorption (Pb) and Cadmium (Cd) by Flavobacterium Sp Bacteria. International
Journal of Scientific and Technology Research, Volume 8(11), pp. 3611–3615
Vendruscolo, F., da Rocha Ferreira, G.L.,
Antoniosi Filho, N.R., 2017. Biosorption of Hexavalent Chromium by
Microorganisms. International Biodeterioration & Biodegradation, Elsevier Volume 119pp. 87–95
Wilan, T., Lieswito, N.A., Suwardi, A., Hadisoebroto, R., Fachrul, M.F., Rinanti, A., 2020. The Biosorption of Copper Metal Ion by Tropical Microalgae Beads Biosorbent. International Journal of Scientific and Technology Research, Volume 9(1), pp. 3533–3536
