Enhancement of Phycocyanin Extraction from Dry Spirulina platensis Powder by Freezing-Thawing Pre-treatment
Corresponding email: abudiman@ugm.ac.id
Published at : 28 Jun 2023
Volume : IJtech
Vol 14, No 4 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i4.5169
Sulistiawati, E., Rochmadi, R., Hidayat, M., Budiman, A., 2023. Enhancement of Phycocyanin Extraction from Dry Spirulina platensis Powder by Freezing-Thawing Pre-treatment. International Journal of Technology. Volume 14(4), pp.780-790
| Endah Sulistiawati | 1. Chemical Engineering Department, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika 2, Yogyakarta 55281, Indonesia, 2. Chemical Engineering Department, Faculty of Industrial Technology, |
| Rochmadi | Chemical Engineering Department, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika 2, Yogyakarta 55281, Indonesia |
| Muslikhin Hidayat | Chemical Engineering Department, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika 2, Yogyakarta 55281, Indonesia |
| Arief Budiman | 1. Chemical Engineering Department, Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika 2, Yogyakarta 55281, Indonesia, 2. Center of Excellence for Microalgae Biorefinery, Universitas Gadja |
Phycocyanin
(PC) is a bioactive compound that can function as an antioxidant,
anti-inflammatory, immunomodulatory, and anti-cancer agent. It can act as a
potential material in preventing COVID-19 and curing those suffering from it. Spirulina
platensis (SP) is one of the microalgae rich in proteins and PC. This study
aimed to determine the optimum PC extraction from SP, using distilled water as
solvent through freezing-thawing pre-treatment. The variables set in the
investigation were water content in SP before freezing (24.7-84.9 % wet basis),
soaking time (0.25, 1, 2, and 6 hours), raw materials’ storage period (1-13
months), freezing time (1-141 days), and the (solvent/biomass) ratio (20-440
mL/g). Spirulina platensis powder was soaked, frozen, thawed, and
extracted in batch operation. The residue was extracted with the same solvent.
The PC concentration in the filtrate was determined by measuring its absorbance
using a spectrophotometer at wavelengths 615 and 652 nm. The experiment gave
the optimum yield at a water content of 81.9% (wet basis), soaking time of 6
hours, freezing time of 1 day, and a solvent-to-biomass ratio of 100 mL/g. The
optimum storage period of the raw material was one month. The phycocyanin IC50 value of 1.485 mg/L.
Freezing-thawing pre-treatment; Phycocyanin; Spirulina platensis
The COVID-19 pandemic has encouraged researchers to prevent its spread and treat patients suffering from it. People with comorbidities, namely degenerative diseases, are highly vulnerable to severe symptoms. Before this pandemic, some degenerative diseases, such as heart disease, stroke, and cancer, were the leading causes of death (Ministry of Health RI, 2019). The number of people dealing with cancer increases yearly (Sung et al., 2021; Bray et al., 2018). Phycocyanin is one of the phycobiliproteins and bioactive components in microalgae that functions as an antioxidant (Renugadevi et al., 2018; Dejsungkranont, Chen, and Sirisansaneeyakul, 2017), an immunomodulator (Grover et al., 2021), and an anti-cancer agent (Czerwonka et al., 2018; Hernandez, Khandual. and Lopez, 2017; Pan et al., 2015). It can inhibit inflammation that causes damage to lung tissues (Li et al., 2020). It can also significantly reduce inflammatory levels (Grover et al., 2021; Fernandez-Rojas, Hernandez-Juarez, and Pedraza-Chaverri, 2014). C-phycocyanin strengthens immunity and is safe to consume since it does not trigger acute diseases and sub-chronic toxicities (Grover et al., 2021).
Microalgae are
photosynthetic microorganisms that convert solar energy into chemical energy
through photosynthesis in their chlorophyll. Microalgae can grow in fresh water
and seawater. Microalgae have diverse nutritional content, especially protein,
carbohydrates, and fats (Rosmahadi et al.,
2021). Various microalgae that can function as a food source or energy
include Botryococcus braunii, Chlorella vulgaris, Dunaliella tertiolecta,
Spirulina platensis, and Tetraselmis suecica (Rosmahadi
et al., 2021; Rosli et al., 2020). Spirulina platensis
is one of the microalgae that can be a source of protein (Sela, Budhijanto, and Budiman, 2021; Vernes et al., 2019; Soni, Sudhakar, and
Rana, 2017). It is
preferable due to its easiness of being cultivated in fresh water. The content
of PC in SP varies from 5 to 20% (Garcia and Mejia,
2021). Consuming Spirulina or phycocyanobilin-enriched Spirulina
extracts may potentially boost type 1 interferon response in the circumstances
of RNA viral infection (McCarty and DiNicolantonio,
2020). Phycocyanin isolation begins with the cell wall breaking. The
bioactive substances inside the cell can get out more quickly through the
broken cell wall so that the extraction of PC becomes fast. If the cell wall
remains intact, the extraction will be prolonged because the molecules have to
diffuse through it. In general, microalgae cell walls are pretty strong, thus
requiring an extraordinary method to break them down. The success of PC
extraction significantly hinges on this initial step (Chia
et al., 2019).
Various ways of cell wall
breaking have been carried out, including sonication (Dianursanti
et al., 2020; Pratiwi, Utama, and Arbianti, 2020; Pan-utai and Iamtham,
2019; Ilter et al., 2018; Rodrigues et al., 2018; Tavanandi et
al., 2018), microwave (Wang, Zhang, and
Fang, 2019; Ilter et al.,
2018), homogenization with a stirrer (Rodrigues et
al., 2019; Ilter et al., 2018; Tavanandi et al., 2018; Silveira
et al., 2007), freeze-thawing (Chia et
al., 2019; Ilter et al., 2018; Tavanandi et al., 2018),
pulsed electric field (Jaeschke et al., 2019; Martínez et al.,
2017), and high-pressure homogenization of up to 350 bars (Deniz, Ozen, and Yesil-Celiktas, 2016). Phycocyanin is very sensitive to
temperatures above 60°C (Su et al., 2014; Chaiklahan,
Chirasuwan, and Bunnag, 2012; Antelo, Costa,
and Kalia, 2008), so a proper method is needed to extract
it from SP. The disadvantages of conventional methods include the relatively
long stirring time (Rodrigues et al., 2018; Silveira
et al., 2007). The agitation process usually comes into contact
with the ambient air, so the PC’s quality is not good if not immediately stored
at low temperatures.
This study used the
freezing method to break the cell wall. This method is considerable because it
can maintain the quality of the product gained, given that PC is easily damaged
if left at room temperature (or higher) or exposed to ambient air. However,
freezing also causes ice expansion which can break the cell wall due to volume
changes (Dombrovsky et al., 2015).
Therefore, the water amount in Spirulina must be precise to ensure a successful
extraction. A small amount of water in the cell makes the ice expansion
insufficient, thus preventing the cell wall from breaking. Several researchers
have extracted phycocyanin from Spirulina platensis by the
freezing-thawing method at a freezing temperature of -20°C (Prabakaran et al., 2020; Chentir et al.,
2018) or -40°C (Tavanandi et al.,
2018). Phycocyanin yielded 52.82%-62.76% with 4-6 freezing-thawing
cycles (Prabakaran et al., 2020; Tavanandi et
al., 2018). However, repeating freezing-thawing cycles are time and
energy-consuming and only suitable for laboratory scales (Jaeschke et al., 2021). Therefore, it is
necessary to study the freezing-thawing method with only one cycle using a
freezing temperature slightly below 0°C to save energy and obtain satisfactory
extraction results.
In this research, distilled
water used as a solvent and the appropriate water content used in Spirulina
platensis will determine the success of the freezing process. If the
cell lacks water, the expansion of ice inside the cell is not enough to break
down the cell wall. Conversely, excess water will cause it to be outside the
cell. It will freeze both inside the cell and outside the cell. The ice outside
the cell will prevent the cell wall from breaking, thus decreasing the number
of cells broken. As a result, the phycocyanin content will also decrease. This
phenomenon indicates that an appropriate water content allowing the cell wall
to break during freezing is necessary. In this case, the freezing-thawing
method is superior, as it can damage the cell walls and obtain a better quality
of PC produced. Therefore, this research aimed to determine the optimum water
content in freezing SP to get a good extract. The variables studied were
soaking time, the storage period of raw materials, freezing time, and the
solvent-to-biomass ratio.
2.1. Materials
Spirulina platensis powder
was purchased from Nogotirto Algae Park, Yogyakarta, Indonesia. The
content of water, protein, fat, ash, and carbohydrates was determined based on
the proximate analysis (AOAC, 2010). The
solvent used was distilled water.
2.2. Freezing-thawing pre-treatment and
extraction
The researchers prepared several
specimens, each of which contained one gram of SP powder added with various
amounts of distilled water to get different water contents. Each of them was
soaked for 15 minutes, 1, 2, and 6 hours, and then let to freeze. After 24
hours of freezing, they were thawed and then added with distilled water for
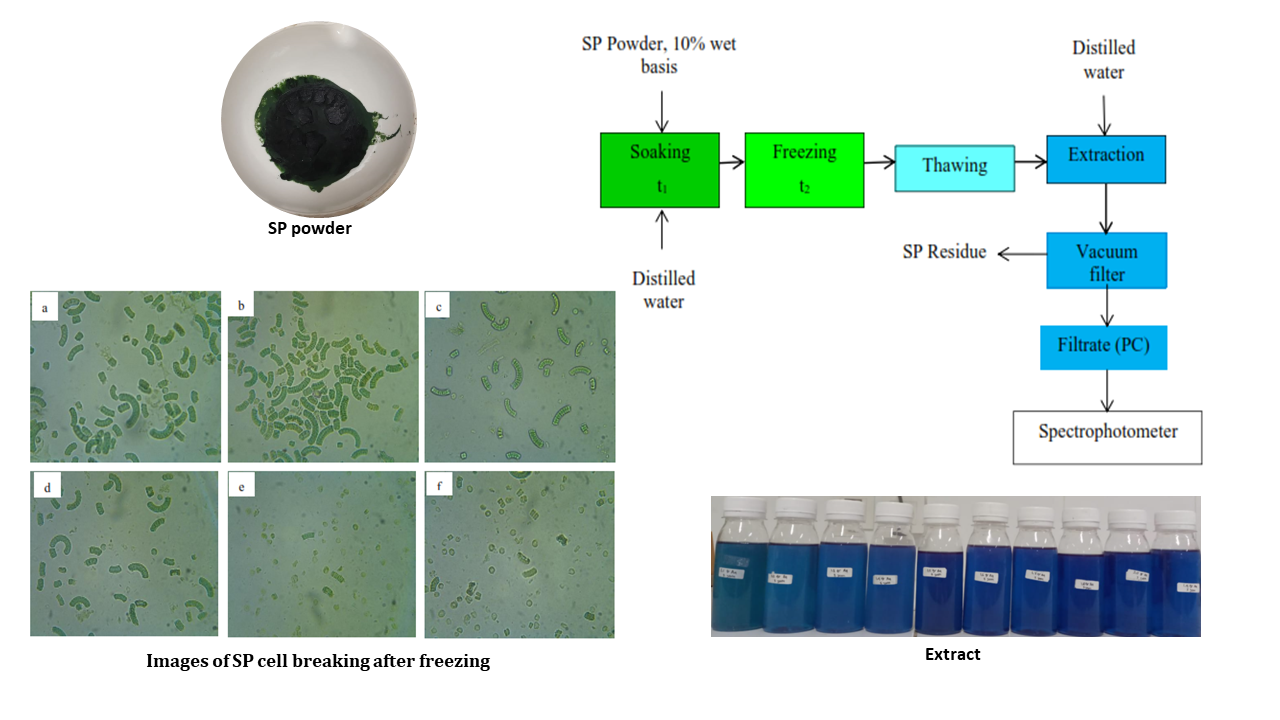
extraction using a vacuum filter. The absorbance was measured using a
spectrophotometer. Figure 1 depicts the experimental procedures (where t1
was soaking time, and t2 was freezing time).
Figure 1 Experimental
procedure of PC extraction from SP powder by the freezing-thawing method
2.3. The equilibrium of solid-liquid extraction
Experiments on solid-liquid extraction equilibrium were carried out in batches, in which the residue from the first extraction was added with pure solvent (distilled water). After filtering, the second residue was added with distilled water and then filtered. The extraction was complete when no phycocyanin was found in the extract, as indicated by the absorbance at a wavelength of 652 nm near zero.
2.4. Phycocyanin determination
The concentration of phycocyanin in the
filtrate was determined using a spectrophotometer at 615 nm and 652 nm, with
the following equations (1) to (4) (Rodrigues et
al., 2019; Pan-utai and Iamtham, 2019; Rodrigues et al., 2018; Deniz
Ozen, and Yesil-Celiktas, 2016;
Silveira et al., 2007).
where CPC was the concentration of
chloro-phycocyanin (g/L), APC was the concentration of allophycocyanin
(g/L), PCt was total phycocyanin (g/L), OD615 was the
filtrate’s optical density at 615 nm, and OD652 was the
filtrate’s optical density at 652 nm from a spectrophotometer.
The yield of
phycocyanin (mg/g) was:
where V was the
solvent volume (mL), and DB (dry basis) was the mass of SP powder (g).
2.5. Antioxidant activity
Antioxidant activity was investigated by
DPPH (2,2-Diphenyl-1-picrylhydrazyl) radical scavenging activity. Spirulina
platensis (water content 80%) 100 mg dissolved in 5 mL of ethanol p.a. The
sample was extracted using sonication for 15 minutes, then filtered. Put the
filtrate in a 10 mL volumetric flask, add ethanol to 10 mL, and mix
homogeneously. We prepared various solutions concentrations of ethanolic
extract. One mL of the sample was mixed with 1 mL of 0.15 mM DPPH in absolute
ethanol. The mixtures were then incubated at room temperature for 30 mins in
the dark. A spectrophotometer measured the absorbance at 517 nm to monitor the
DPPH radical decrease. The IC50 was de?ned as the concentration of
ethanolic extract of phycocyanin to scavenge 50% initial DPPH radical, and it
was re?ected by a 50% reduction of absorbance (Abdullah
et al., 2020; Pan-utai and Iamtham, 2019).
Based on the proximate analysis, Spirulina
platensis contained 9.57% water, 39.77% protein, 0.8% fat, 7.12% ash, and
42.76% carbohydrates (by difference). Phycocyanin can function as an
immunomodulator, reduce inflammatory level, and strengthens immunity. It does
not trigger acute diseases and sub-chronic toxicity (Grover
et al., 2021). According to D’Alessandro
and Filho (2016), the structure of phycocyanin is shown in Figure 2.
Figure 2 The structure of
phycocyanin
3.1. Effect of water content and soaking
time
Figure 3 shows the effects of water
content and soaking time on the extraction yield. Having the same water
contents, the longer the soaking time, the higher the PC content.
Figure 3 Effects of water content
and soaking time on the extraction yield
The
longer the soaking time, the more sufficient the duration taken by the
distilled water added to SP powder to diffuse into the biomass pores. If the
water is already inside the cell before freezing, when it freezes, its phase
inside the cell will change, its volume will expand, and the formed ice will
break down the cell wall. Consequently, PC will quickly come out during the
thawing and extraction stages. The more the cell walls are broken, the higher the
PC content. When the soaking time was 15 minutes, the yield would be below 10%.
For 15 minutes, not all of the water added to the biomass could enter the cell.
At a water content of 81.9 %, the yield obtained was below those when the
soaking times were 1, 2, and 6 hours. Of all the experiments, the highest PC
content of 81.65% was obtained when after soaked for 6 hours before being
frozen. Microscopic visualization of the cells after freezing (with soaking
times of 1 and 6 hours before freezing) is present in Figure 4.
Figures 4 Microscopic images
of the cells after freezing at a water content of 81.8 % wet basis: a) soaked
for 1 hour; b) soaked for 6 hours
Figures
4 show that the six-hour soaking made the number of cells broken more than the
one-hour one did. Figure 4b indicates that in the six-hour soaking, the amount
of water diffusing into the cells was more than that in the one-hour hone
(Figure 4a), increasing the number of cells broken significantly. The
microscopic images of the effect of water content on the number of cells broken
are shown in Figure 5.
Figures 5 Images of SP cell
breaking after freezing: a) Initial (non-freezing), b) X=54.8 %, c) X=69.9 %,
d) X=77.4 %, e) X=81.9 %, f) X=84.9 % wet basis
Figure
5a shows the initial SP powder condition before freezing. Figures 5b, 5c, and
5d show that after freezing at a water content of less than 80%, the cells’
states were relatively the same as their initial state. These data show that
water content below 80% is insignificant for breaking down cell walls. Figures
5e and 5f indicate higher numbers of cells broken.
3.2. Effect of raw
materials storage period
The raw materials storage period also
affects the extraction yield. It can be seen in Figure 6 that the longer the
storage period of raw materials, the lower the yield. For the same soaking time
(6 hours), at the one-month storage period (T6-S1), the highest yield was
around 80%, while at the four-month storage period (T6-S4), the yield was 59%.
Figure 6 Effect of soaking
time and storage period of raw SP on yield PC
For
a storage period of 12-13 months, the maximum yield was 30%. It was probably
due to the decrease in PC content in the raw material (possibly due to
oxidation), so the extracted product’s PC content also decreased.
3.3. Effect of freezing time
The effect of freezing on the total PC is
described in Figure 7. Theoretically, the freezing time would not affect the
resulting extract for the same water content. At the water content of 80%, freezing
time of 1 day and 13 days resulting in the optimum of total phycocyanin.
Figure 7 Effect of freezing
time on total phycocyanin extracted
Figure
7 shows that the water contents, ranging from 40% to 60%, resulted in the
relatively same total phycocyanin for freezing times 1 and 24 days. Likewise,
at water contents above 80%, there were no significant differences between
those after freezing for 140 and 141 days. It seemed that the freezing time did
not affect the phycocyanin content if the frozen state had been reached, but it
could be explored more to find the optimum freezing time.
3.4. Effect of (Solvent/Biomass) ratio
The solvent-to-SP biomass ratio was also
studied in the extraction step, as presented in Figure 8.
Figure 8 Effect of the ratio
of solvent to biomass on the yield
The higher the solvent-to-biomass ratio, the higher the yield (PC produced). After the S/B ratio = 200, the yields were relatively fixed, meaning the extraction was close to equilibrium. The higher the S/B ratio, the lower the PC concentration in the extract because the raw biomass content did not change. The previous researcher obtained a maximum yield of 74.51 mg/g (dry biomass) with a purity of 0.56 at a ratio of S/B of 10 after four cycles (Tavanandi et al., 2018). In this study, the ratio of S/B of 100 with one freezing-thawing cycle yielded a maximum PC of 84.69 mg/g (dry SP). Compared to Tavanandi et al. (2018), this study used one freezing-thawing cycle, requiring less energy than four cycles (and a freezing temperature of -40°C). This method is more applicable for scaling up (using the freezing temperature of 0 to -2°C). Another previous researcher operating the ultrasonic extraction method found the highest concentration of phycobiliprotein with a mixed solvent containing N-methyl-2-hydroxyethyl ammonium acetate and N-methyl-2-hydroxyethyl ammonium format (2-HEAA+2-HEAF) at pH 6.5 with (S/B) 7.93 mL/g. The extract contained CPC and APC of 5.95 mg/g and 6.34 mg/g, respectively (Rodrigues et al., 2018). The complete comparison of research results is presented in Table 1.
Table 1 Comparison of the PC
extraction results from SP with those in other studies
|
The extraction method and Solvent |
S/B, mL/g |
Yield, mg/g |
References |
|
Four
freezing-thawing cycles, distilled water |
10.00 |
74.51 |
Tavanandi et al., 2018 |
|
Freezing,
CaCl2 1.5% |
100.00 |
55.33 |
Ilter et al., 2018 |
|
Ultrasonic,
2-HEAA+2-HEAF |
7.93 |
12.29 |
Rodrigues et al., 2018 |
|
Mechanical
agitation, 2-HEAA+2-HEAF |
6.59 |
22.07 |
Rodrigues et al., 2019 |
|
Freeze-dried
and homogenization, 0.01M Sodium phosphate buffer (pH 7.0) |
25.00 |
78.17 |
Pan-utai and Iamtham, 2019 |
|
One
freezing-thawing cycle, distilled water |
100.00 |
84.69 |
This
study |
Phycocyanin extraction from SP occurs
rapidly. Assumably, the extraction reaches equilibrium in the same manner.
Phycocyanin is very soluble in water. After thawing, the extraction stage takes
place quickly. In this study, the experiments were carried out in batches, in
which the residue from the first extraction was added with pure solvent
(distilled water) and filtered again until completed. The extraction was
complete if there was zero phycocyanin content in the extract. The extract was
initially blue and became brighter after the next process, as shown in Figure
9.
Figure 9 Phycocyanin from SP: a) images of extracts, b) PC concentrations
3.5. Antioxidant
activity
Antioxidant activity is
represented by the IC50 value, namely the concentration of the
solution sample required to inhibit 50% of DPPH free radicals. The IC50
value of CPC from Oscillatoria
tenuis was 1.75 mg/mL, while Ascorbic acid was
0.015 mg/mL (Thangam et al., 2013). The concentration of CPC extracted from 0.01 g/mL of SP was 2.418
mg/L, achieving an IC50 value of 1.485 mg/L. The previous researcher
achieved the optimum scavenging activity (52.13 % inhibition) of freeze-dried
SP under homogenization of 0.02 g/mL biomass concentration (0.01 M solvent
concentration). The concentration of CPC was 1.67 mg/mL (Pan-utai and Iamtham, 2019).
The
water content in the biomass affected PC extraction from SP by freezing-thawing
pre-treatment. The addition of distilled water to dry SP until the optimum
water content of 81.9 % (wet basis) with soaking time for 6 hours resulted in a
yield with a percentage of 81.65 %. If the water contents (before freezing)
were the same, the freezing time did not affect the extraction results. The
greater the solvent-to-biomass ratio, the greater the yield obtained until
equilibrium. The optimum freezing time was 81.9 % (wet basis), the raw material
storage period was one month, and the solvent-to-biomass ratio was 100 mL/g,
and the IC50 of 1.485 mg/L. We recommend measuring the temperature
changing from room to freezing temperature using a data logger and modeling the
freezing zone during phase changes.
This
work was partly supported by a research grant from Universitas Ahmad Dahlan,
Yogyakarta, Indonesia (PDD-335/SP3/LPPM-UAD/VI/2021).
Abdullah, N.A., Zullki?ee, N., Zaini,
S.N.Z., Taha, H., Usman, A., 2020. Phytochemicals, Mineral Contents,
Antioxidants, and Antimicrobial Activities of Propolis Produced by Brunei Stingless
Bees Geniotrigona Thoracica, Heterotrigona Itama, and Tetrigona Binghami. Saudi
Journal of Biological Sciences, Volume 27, pp. 2902–2911
Antelo, S.F., Costa, J.A.V., Kalia, S.J.,
2008. Thermal Degradation Kinetics of The Phycocyanin from Spirulina
Platensis. Biochemical Engineering Journal, Volume 41, pp. 43–47
AOAC., 2010. Official Methods of
Proximate Analysis. AOAC International, Gaithersburg, Maryland.,
p.15
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R.I., Torre, L.A.,
Jemal, A., 2018. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence
and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer Journal for Clinicians, Volume 68, pp. 394–424
Chaiklahan, R., Chirasuwan, N., Bunnag, B., 2012. Stability of Phycocyanin
Extracted from Spirulina Sp.: Influence of Temperature, pH and Preservatives. Process. Biochemistry, Volume 47, pp. 659–664
Chentir,
I., Hamdi, H., Li, S., Doumandji, A., Markou, G.,
Nasri, M.,
2018. Stability, Bio-Functionality and Bio-Activity of
Crude Phycocyanin from a Two-Phase Cultured Saharian Arthrospira Sp. Strain. Algal
Research, Volume 35, pp. 395–406
Chia, S.R., Chew, K.W., Show., P.L., Xia, A., Ho, S., Lim, J.W.,
2019. Spirulina Platensis Based Biorefinery for The Production of Value-Added
Products for Food and Pharmaceutical Applications. Bioresource Technology,
Volume 289, 121727
Czerwonka, A., Ka?awaj, K., Slawinska-Brych, A., Lemieszek, M.K.,
Bartnik, M., Wojtanowski, K.K., Zdzisinska, B., Rzeski, W., 2018. Anticancer Effect
of The Water Extract of A Commercial Spirulina (Arthrospira Platensis) Product on
The Human Lung Cancer A549 Cell Line. Biomedicine
& Pharmacotherapy, Volume 106, pp. 292–302
D’Alessandro, E.B., Filho, N.R.A., 2016. Concepts and Studies on
Lipid and Pigments of Microalgae: A Review. Renewable and Sustainable Energy Reviews, Volume, 58, pp. 832–841
Dejsungkranont, M., Chen, H., Sirisansaneeyakul, S., 2017.
Enhancement of Antioxidant Activity of C-Phycocyanin of Spirulina Powder
Treated with Supercritical Fluid Carbon Dioxide. Agriculture and Natural Resources, Volume 51, pp. 347-354
Deniz, I., Ozen, M.O., Yesil-Celiktas,
O., 2016. Supercritical Fluid Extraction of Phycocyanin and Investigation of
Cytotoxicity on Human Lung Cancer Cells. The Journal of Supercritical Fluids,
Volume 108, pp. 13–18
Dianursanti, Siregar, A.R., Maeda, Y., Yoshino, T.,
Tanaka, T., 2020. The Effects of Solvents
and Solid-to-Solvent Ratios on Ultrasound-Assisted Extraction of Carotenoids
from chlorella vulgaris. International Journal of Technology,
Volume 11(5), pp. 941-950
Dombrovsky,
L.A., Nenarokomova, N.B., Tsiganov, D.I., Zeigarnik, Y.A., 2015. Modeling of Repeating
Freezing of Biological Tissues and Analysis of Possible Microwave Monitoring of
Local Regions of Thawing. International
Journal of Heat and Mass Transfer, Volume 89, pp. 894–902
Fernandez-Rojas,
B., Hernandez-Juarez, J., Pedraza-Chaverri, J., 2014. Nutraceutical Properties
of Phycocyanin. Journal of Functional Foods, Volume 11, pp. 375–392
Garcia, H.F.L., Mejia, N.L., 2021. Mathematical Model of a
Bubble Column for The Increased Growth of Arthrospira Platensis and The
Formation of Phycocyanin. International Journal of Technology, Volume
12(2), pp. 232–242
Grover, P., Bhatnagar, A.,
Kumari, N., Bhatt, A.N., Nishad, D.K., Purkayastha, J., 2021. C-Phycocyanin-A
Novel Protein from Spirulina Platensis -in Vivo Toxicity, Antioxidant
and Immunomodulatory Studies. Saudi Journal of Biological Sciences, Volume 28, pp.
1853–1859
Ilter, I., Akyil, S., Demirel, Z., Koc., M., Conk-Dalay, M.,
Kaymak-Ertekin, F., 2018. Optimization of Phycocyanin Extraction from Spirulina
Platensis Using Different Techniques. Journal of Food Composition and Analysis,
Volume 70, pp. 78–88
Jaeschke, D.P., Mercali, G.D., Marczak, L.D.F., Müller, G., Frey,
W., Gusbeth, C., 2019. Extraction of Valuable Compounds from Arthrospira Platensis
Using Pulsed Electric Field Treatment. Bioresource
Technology, Volume 283, pp. 207–212
Jaeschke, D.P., Teixeira, I.P, Marczak,
L.F., Mercali,
G.D., 2021. Phycocyanin
from Spirulina: A Review of Extraction Methods and Stability. Food Research International, Volume 143, 110314
Li,
W., Lu, L., Liu, B., Qin, S., 2020. Effects of Phycocyanin on Pulmonary
and Gut Microbiota in a Radiation-Induced Pulmonary Fibrosis Model. Biomedicine & Pharmacotherapy, Volume 132, p. 110826
Martínez, J.M., Luengo, E.,
Saldaña, G., Alvarez, I., Raso, J., 2017. C-Phycocyanin Extraction Assisted by
Pulsed Electric Field from Artrosphira Platensis. Food Research International, Volume
99, pp. 1042–1047
McCarty,
M.F., DiNicolantonio, J.J., 2020. Nutraceuticals have Potential for Boosting
The Type 1 Interferon Response to RNA Viruses Including In?uenza and
Coronavirus. Progress in
Cardiovascular Diseases, Volume 63, pp. 383–385
Ministry
of Health Republic of Indonesia, 2019. Profil Kesehatan Indonesia
Tahun 2018 (Indonesia Health Profile 2018). Ministry of Health Republic of Indonesia,
p. 244
Pan,
R., Lu, R., Ying Zhang, Y., Zhu, M., Zhu, W., Yang, R., Zhang, E., Ying, J.,
Teng Xu, T., Yi, H., Jinsong Li, J., Shi, M., Xu, Z., Li, P., Qiyu Bao, Q.,
2015. Spirulina Phycocyanin Induces Differential Protein Expression and
Apoptosis in SKOV-3 Cells. International Journal of Biological
Macromolecules, Volume 81, pp. 951–959
Pan-utai,
W., Iamtham, S., 2019. Extraction, Puri?cation
and Antioxidant Activity of Phycobiliprotein from Arthrospira Platensis.
Process Biochemistry, Volume 82, pp. 189–198
Prabakaran, G., Sampathkumar, P., Kavisri, M., Moovendhan,
M., 2020. Extraction and Characterization of Phycocyanin from Spirulina Platensis and
Evaluation of Its Anticancer, Antidiabetic and Antiin?ammatory Effect. International
Journal of Biological Macromolecules, Volume
153, 256–263
Pratiwi, F.A., Utami, T.S., Arbianti, R., 2020. Using Ultrasonic
Assisted Extraction to Produce a Bioinsecticide from Cigarette Butt Waste and
Green Solvent to Control Armyworm Infestation.
International Journal of Technology, Volume 11(7), pp. 1329-1336
Renugadevi, K., Nachiyar,
C.V., Sowmiya, P., Sunkar, S., 2018. Antioxidant Activity of Phycocyanin
Pigment Extracted from Marine Filamentous Cyanobacteria Geitlerinema Sp
TRV57. Biocatalysis and Agricultural
Biotechnology, Volume 16, pp. 237–242
Rodrigues, R.D.P., de Castro,
F.C., de Santiago-Aguiar, R.S., Rocha, M.V.P., 2018. Ultrasound-Assisted
Extraction of Phycobiliproteins from Spirulina (Arthrospira) Platensis
Using Protic Ionic Liquids as Solvent. Algal
Research, Volume 31, pp.454–462
Rodrigues, R.D.P., de Lima,
P.F., de Santiago-Aguiar, R.S., Rocha, M.V.P., 2019. Evaluation of Protic Ionic
Liquids as Potential Solvents for The Heating Extraction of Phycobiliproteins from
Spirulina (Arthrospira) Platensis. Algal
Research, Volume 38, p. 101391
Rosli, S.S., Kadir, W.N.A.,
Wong, C.Y., Lim, J.W., Lam, M.K., Yusup, S., Kiatkittipong, W., Kiatkittipong, K.,
Usman, A., Han, F.Y., 2020. Insight Review of Attached Microalgae Growth
Focusing on Support Material Packed in Photobioreactor for Sustainable
Biodiesel Production and Wastewater Bioremediation. Renewable and
Sustainable Energy Reviews, Volume 134, p. 110306
Rosmahadi,
N.A., Leong, W.H., Rawindran, H., Ho, Y.C., Mohamad, M., Ghani, N.A., Bashir,
M.J.K., Usman, A., Lam, M.K., Lim, J.W., 2021. Assuaging Microalgal Harvesting
Woes Via Attached Growth: A Critical Review to Produce Sustainable Microalgal
Feedstock. Sustainability, Volume 13, p. 11159
Sela, K., Budhijanto, W.,
Budiman, A., 2021. Protein Extraction from Spirulina Platensis by Using
Ultrasound Assisted Extraction: Effect of Solvent Types and Extraction Time. Key
Engineering Materials, Volume 872, pp. 33–37
Silveira, S.T., Burkert,
J.F.M., Costa, J.A.V., Burkert, C.A.V, Kalil, S.J., 2007. Optimization of Phycocyanin
Extraction from Spirulina Platensis Using Factorial Design. Bioresource
Technology, Volume 98(8), pp. 1629–1634
Su, C.H., Liu, C.S., Yang,
P.C., Syu, K.S., Chiuh, C.C., 2014. Solid-Liquid Extraction of Phycocyanin from
Spirulina Platensis: Kinetic Modeling of Influential Factors. Separation and
Purification Technology, Volume 123, pp. 64–68
Sung, H., Ferlay, J., Siegel,
R.L., Laversanne, M.,
Soerjomataram, I., Jemal, A., Bray, F., 2021. Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185. CA Cancer Journal for
Clinicians, Volume 71, pp. 209–249
Tavanandi, H.A., Mittal, R., Chandrasekhar, J., Raghavarao,
K.S.M.S., 2018. Simple and Efficient Method for Extraction of C-Phycocyanin from
Dry Biomass of Arthospira Platensis. Algal
Research, Volume 31, pp. 239–251
Thangam, R., Suresh, V., Princy, W.A.,
Rajkumar, M., SenthilKumar, N., Rengasamy, R., Anbazhagan, C., Kaveri, K.,
Kannan, S., Gunasekaran, P., 2013. C-Phycocyanin from Oscillatoria Tenuis
Exhibited an Antioxidant and in Vitro Antiproliferative Activity Through
Induction of Apoptosis and G0/G1 Cell Cycle Arrest. Food
Chemistry, Volume 140, pp. 262–272
Vernes, L., Abert-Vian, M., El Maataoui, M,
Tao, Y., Bornard, I., Chemat, F., 2019. Application of Ultrasound for Green
Extraction of Proteins from Spirulina: Mechanism, Optimization, Modeling, and
Industrial Prospects. Ultrasonics – Sonochemistry, Volume 54, pp. 48–60
Wang, J., Zhang, M., Fang, Z., 2019. Recent Development in
Efficient Processing Technology for Edible Algae: A Review. Trends in Food Science &Technology,
Volume 88, pp. 251–259
