A New Multi-Component Solid of Atorvastatin Calcium with a Dipicolinic Acid Coformer for Improving the Water Solubility
Corresponding email: yudi.farmasi@unej.ac.id
Published at : 09 May 2023
Volume : IJtech
Vol 14, No 3 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i3.5088
Wicaksono, Y., Nugraha, A.S., Irawan, E.D., Nada, N., Nurhansyah, S.D., 2023. A New Multi-Component Solid of Atorvastatin Calcium with a Dipicolinic Acid Coformer for Improving the Water Solubility. International Journal of Technology. Volume 14(3), pp. 618-627
| Yudi Wicaksono | Drug Modification Research Group, Faculty of Pharmacy, University of Jember, Jl. Kalimantan No. 37, Jember 68121, Indonesia |
| Ari Satia Nugraha | Drug Utilisation and Discovery Research Group, Faculty of Pharmacy, University of Jember, Jl. Kalimantan No. 37, Jember 68121, Indonesia |
| Eka Deddy Irawan | Drug Delivery Research Group, Faculty of Pharmacy, University of Jember, Jl. Kalimantan No. 37, Jember 68121, Indonesia |
| Nadifa Nada | Drug Modification Research Group, Faculty of Pharmacy, University of Jember, Jl. Kalimantan No. 37, Jember 68121, Indonesia |
| Sofyan Dimas Nurhansyah | Drug Modification Research Group, Faculty of Pharmacy, University of Jember, Jl. Kalimantan No. 37, Jember 68121, Indonesia |
Atorvastatin
calcium is a potent active
pharmaceutical ingredient that reduces blood cholesterol
levels. However, oral bioavailability is low because it is difficult to
dissolve in water. Therefore, further research to increase the solubility of
the drug is needed to improve its bioavailability properties. This research aimed
to increase the solubility of atorvastatin calcium through the formation of a
multi-component solid. Furthermore, the drug was formed into a multi-component
solid with dipicolinic acid as a coformer using the solvent
evaporation-quenching method. A powder X-ray diffractometer (PXRD), Fourier
transform infrared (FTIR) spectrometer, differential scanning calorimeter
(DSC), and scanning electron microscope (SEM) was used to characterize the
solid. Also, the shake-flask method was used to analyze the solubility of the
solid in distilled water. The results showed that atorvastatin calcium and
dipicolinic acid formed a new multi-component solid of cocrystal type. The solubility of the atorvastatin calcium from the
multi-component solid was 344.31 ± 37.09 mg/L, which was a significant increase
of about 2.7-fold compared to the pure form.
Atorvastatin calcium; Dipicolinic acid; Multi-component solid; Solubility properties
The
rate of drug absorption in the gastrointestinal tract is determined by
solubility. Orally administered drugs must dissolve in the gastrointestinal
fluid for a passive diffusion process, the main absorption mechanism, to occur (Pindelska, Sokal, and Kolodziejski, 2017;
Maggi et al., 2015). The bioavailability of the absorbed drug fraction
produced the desired pharmacological effect (Williams et al., 2013). Therefore, drugs with low
solubility levels have oral bioavailability problems, posing challenges and
opportunities for their development (Javeer,
Patole, and Amin, 2013; Kawabata et al., 2011).
Atorvastatin calcium is a
potent statin drug that reduces blood cholesterol levels. It works by
inhibiting the enzyme HMG-CoA reductase in cholesterol biosynthesis (Anwar,
Warsi, and Mallick, 2011). According to the
biopharmaceutical classification system, it is categorized as a Class II drug with high
permeability but low solubility. The bioavailability of oral preparations is
about 12% due to their low solubility property (Anwar, Warsi, and Mallick, 2011). Therefore, research to increase the solubility of
this drug is needed.
The multi-component solid is one technique used to
improve the solubility of poorly soluble drugs (Zaini et
al., 2020; Chadha, Sharma, and Haneef, 2017; Haneef and Chadha, 2017). Cocrystal is a type of multi-component solid that
has gained attention for improving the solubility properties of poorly soluble
drugs. Furthermore, the
multi-component cocrystal is formed through noncovalent intermolecular
interactions between drug molecules and coformers to produce a single-phase
material with a new crystal lattice structure. Generally, the drugs that form cocrystals have new physical properties
that differ significantly from the starting materials (Shaikh
et al., 2018). The solubility of
the multi-component cocrystal
can be improved by
lowering the lattice energy and/or increasing the solvent affinity (Thakuria et al., 2013). Also,
the presence of a diverse coformer in the multi-component solid increases the
possibility of obtaining drug solids with better physical solubility properties
(Douroumis, Ross, and Nokhodchi, 2017; Gao et al., 2012; Tilborg et al., 2010). In addition, ketoprofen-nicotinamide, atorvastatin
calcium-succinic acid, and aripiprazole-succinic acid are examples of
multi-component cocrystals shown to increase solubility (Butreddy
et al., 2021; Wicaksono et al., 2019; Wicaksono et al.,
2018).
The research aimed to improve the
water solubility properties of atorvastatin calcium through the formation of a
multi-component solid. Dipicolinic acid was used as a coformer due to its
solubility in water. The molecules have two donors and five acceptors of
hydrogen bonds, making it easy to form intermolecular interactions with other
molecules (Hiendrawan et al., 2016). In addition, the dipicolinic acid coformer is inert.
Hence it has no toxic effects. The atorvastatin calcium-dipicolinic acid
multi-component solid was prepared using the solvent evaporation-quenching
method. A powder X-ray diffractometer (PXRD), Fourier transform infrared (FTIR)
Spectrometer, differential scanning calorimeter (DSC), and scanning electron
microscope (SEM) were used to characterize the solid. The shake-flask method
was used to analyze the solubility of the samples in distilled water.
2.1. Materials
Atorvastatin
calcium (>98.0%) was provided by PT Dexa Medica (Palembang, Indonesia).
Dipicolinic acid (>98.5%), methanol (>99.8%), and distilled water were
purchased from Sigma-Aldrich (Saint Louis, USA), PT Smart Lab (Tangerang,
Indonesia) and CV Makmur Sejati (Jember, Indonesia) respectively.
2.2.
Preparation of Multi-component
Solid
The solvent evaporation-quenching method was used to
prepare the atorvastatin calcium-dipicolinic acid multi-component solid. The
preparation of the solid was carried out in a methanol solution with a molar
ratio of atorvastatin calcium-dipicolinic acid (1:1). The solution was
evaporated on a hotplate at 60 °C and stirred at 150 rpm until a dry solid was
obtained. Then the resulting solid was quenched at -80 °C for 12 hours, crushed
with the mortar, and passed through an 80-mesh sieve. The solid powder was stored
in a desiccator until it was tested for
solid-state and solubility properties. The scheme for the preparation of multi-component solids is shown in Figure
1.
2.3. Characterization
2.3.1.
PXRD
PXRD was a method used to analyze the phase types of solid materials. Furthermore, the analysis was conducted using a Philip Xpert diffractometer and a CuK?1 radiation source. The diffractogram was determined by filling the sample holder cavity of the X-ray diffractometer with sample powder and flattening it with a spatula. Also, the X-ray diffractometer was set at a voltage of 40 kV, a current of 30 mA, and a scan speed of 2? = 10°/minute with a range of 2? at 5-50°.
Figure 1 Scheme for
preparation of multi-component solids
2.3.2. DSC
Generally, the DSC test was used to determine the
solid-liquid or solid-solid transformation temperature of the sample. The
Thermo plus EVO DSC 8230 equipment was used, and about 2 mg of the powder
sample was placed into an aluminum container and tightly closed with a press device.
Then the sample was placed into the DSC, which was set to run at a temperature
range of 30-300 °C. The heating rate of DSC was 10 °C/minute under dry nitrogen
gas flow conditions at 50 mL/minute.
2.3.3.
FTIR
The FTIR testing was used to identify the functional
groups and intermolecular interactions of molecules in the solids. About 5 mg
of the sample powder was placed on the equipment’s board (Alpha Bruker), and
then the instrument was run at the wavenumber range of 4000-600 cm-1.
2.3.4. SEM
The SEM analysis aims to obtain the microscopic
properties, including surface topography and particle size of a sample. About 2
mg of the sample was placed on a stub specimen that had been given adhesive and
then coated with platinum for 20 seconds using the Hitachi E-1045 ion sputter.
Then the sample was inserted into the SEM base holder, and the voltage and
current were set at 15 kV and 12 mA, respectively. The observation of the
shape, size, and topography of the sample particles was carried out using the
appropriate magnification.
2.4. Solubility Testing
3.1. The Multi-component Solid
The solvent
evaporation-quenching method was used to prepare an atorvastatin
calcium-dipicolinic acid multi-component solid. Methanol was used as the
solvent because it easily dissolves atorvastatin calcium and dipicolinic acid.
The drug and coformer used in the preparation of the multi-component solid were
0.968 and 0.135 g, respectively, while the total solvent was 15 mL. Also, the
time required for the solvent evaporation process was 60 minutes. The solids
were quenched in a deep freezer at -80 ?C for 12 hours, then crushed with a
mortar and passed through an 80-mesh sieve.
3.2. PXRD Diffractogram
The PXRD characterization
was conducted to analyze the solid-state phase of the atorvastatin
calcium-dipicolinic acid multi-component solid. Although the diffractogram of
crystalline solids is characterized by the presence of sharp diffraction peaks,
that of amorphous solids is characterized by the absence of diffraction peaks.
The formation of new crystalline solids from the starting material mixture was
indicated by a diffractogram with new diffraction peaks different from each
starting material (Karagianni,
Malamatari, and Kachrimanis, 2018).
Figure 2
shows the diffractograms of atorvastatin calcium, dipicolinic acid, and
atorvastatin calcium-dipicolinic acid multi-component solid. The diffractogram
of atorvastatin calcium has diffraction peaks of 2? at 9.4; 10.2; 11.8; 17.0;
19.4; 21.6; and 23.7°, while the dipicolinic acid has diffraction peaks of 2?
at 11.1; 16.9; 19.4; 24.4; 27.9; 32.6; and 37.1°. Also, the physical mixture of
atorvastatin calcium and dipicolinic acid powder showed a diffractogram with
diffraction peaks of 2?, which are a combination or overlay of the respective
diffraction peaks. However, the atorvastatin calcium and dipicolinic acid
multi-component solid showed the new diffraction peaks of 2? at 13.2; 17.9;
18.7; 23.5; and 26.3°, in contrast to the individual peaks.
Figure 2
Diffractogram of (a) atorvastatin calcium, (b) dipicolinic acid, (c) physical
mixture of atorvastatin calcium and dipicolinic acid, and (d) atorvastatin
calcium - dipicolinic acid multi-component solid
The results
of PXRD characterization showed that the solid-state of atorvastatin
calcium-dipicolinic acid multi-component solid has a diffractogram different
from each starting material, indicating the formation of a solid with a new
crystalline phase. Also, the dipicolinic acid molecules acted as a coformer in
the atorvastatin calcium-dipicolinic acid multi-component solid, forming a new
crystal lattice with the atorvastatin calcium molecules (Karagianni, Malamatari, and Kachrimanis, 2018). This lattice has different energy from the starting
material, resulting in new physicochemical properties (Thakuria
et al., 2013).
3.3. DSC Thermogram
DSC
characterization was used to observe the solid-state phase transformation of
the sample due to heating (Wicaksono et al.,
2020). The melting point data,
enthalpy of fusion, and phase transformation profile were obtained from this
characterization (Pindelska, Sokal, and
Kolodziejski, 2017; Karagianni et al., 2018; Qiao et al., 2011). Figure 3 showed the DSC thermograms of atorvastatin
calcium, dipicolinic acid, and atorvastatin calcium-dipicolinic acid
multicomponent solid. The atorvastatin calcium DSC thermogram showed two sharp
endothermic peaks at 155.0 °C (?H = 69.04 J/g) and 236.2 °C (?H = 23.56 J/g),
indicating the melting point and decomposition temperature, respectively (Shete et al., 2010). In
addition, the dipicolinic acid DSC thermogram showed one sharp endothermic peak
at 260.4 °C (?H = 496.76 J/g), indicating the melting point and no
decomposition up to 300 °C which is in line with the literature (Hiendrawan et al., 2016).
The DSC thermogram of atorvastatin
calcium and dipicolinic acid multi-component solid showed two sharp endothermic
peaks at 98.0 °C (?H = 26.62 J/g) and 192.9 °C (?H = 35.21 J/g). The sharp
endothermic peak at 98.0 °C indicated the melting point of the solid, while the
peak at 192.9 °C depicted the decomposition temperature. Therefore, the results of DSC
characterization showed that the solid has a thermogram profile that differs
from the starting material in terms of melting point and decomposition temperatures. The changes in the thermogram profile indicated the
presence of intermolecular interactions in the multi-component solid between
molecules of the drug and the coformer. Also, the multi-component solid of
atorvastatin calcium and dipicolinic acid showed a lower melting point and
enthalpy of melting than the individual material. The decrease in melting point
and enthalpy of melting indicated lower lattice energy, making it easy to
convert the solid into liquid when
heated (Thakuria et al., 2013; Qiao et al.,
2011).
Figure 3 DSC thermogram of (a) atorvastatin calcium, (b)
dipicolinic acid, and (c) atorvastatin calcium - dipicolinic acid
multi-component solid
3.4. FTIR Spectra
The FTIR characterization
aimed to analyze the functional groups in atorvastatin calcium, dipicolinic
acid, and atorvastatin calcium-dipicolinic acid multi-component solids. In
addition, the FTIR spectra were used to analyze the presence of intermolecular
interactions between the drug and the coformer in the multi-component solids (Bekbayeva et al., 2022; Karagianni, Malamatari, and Kachrimanis, 2018; Pindelska Sokal, and
Kolodziejski, 2017). Figure 4 showed the FTIR spectra of atorvastatin calcium, dipicolinic
acid, and atorvastatin calcium-dipicolinic acid multicomponent solid. The drug
showed FTIR spectra with specific absorption peaks at 3364 cm-1
(NH-free stretching), 3096 cm-1 (OH- stretching), 1650 cm-1
(C=O stretching), and 1216 cm-1 (CN stretching), indicating
suitability as stated in the literature (Wicaksono et al.,
2019). According to the literature, the FTIR spectrum of
the coformer showed a wide and strong absorption peak at 3300-2400 cm-1
(OH stretching of the carboxylic acid group) and 1692 cm-1 (C=O
stretching of carboxylic the acid group), respectively (Hiendrawan et al.,
2016).
Figure 4 FTIR
spectra of (a) atorvastatin calcium, (b) dipicolinic acid, and (c) atorvastatin calcium-dipicolinic acid
multi-component solid
The FTIR
spectra of atorvastatin calcium-dipicolinic acid multi-component solid showed
absorption peaks that were the combination of the components. However, the
absorption peaks changed in intensity and wave number. The FTIR spectra showed that the absorption peaks of atorvastatin
calcium in the multi-component solids shifted, with free NH stretching from
3364 to 3381 cm-1, OH from 3096 to 3032 cm-1, C=O from
1650 to 1700 cm-1, and CN from 1216 to 1224 cm-1. Meanwhile, the absorption peaks of dipicolinic acid in
the multi-component solid spectra changed, with a decrease in the intensity of
the O-H absorption peak of the carboxylic acid group and a shift in the C=O
stretching from 1692 to 1648 cm-1. According to the FTIR analysis,
the solid spectra showed shifts in the absorption peaks of the acceptor and
donor groups of hydrogen bonds of atorvastatin calcium and dipicolinic acid
molecules. This indicated the presence of intermolecular hydrogen bonds between
molecules of the drug and coformer in the solid (Karagianni, Malamatari, and Kachrimanis, 2018).
The PXRD analysis showed
that atorvastatin calcium-dipicolinic acid formed a new crystalline solid with
a different diffraction pattern and crystal lattice from the starting
materials. Furthermore, the DSC analysis results showed that the component’s
solid state has a new single solid phase with different thermal properties
(melting point and enthalpy of fusion) from the starting material. The FTIR analysis showed that intermolecular hydrogen
bonds were formed between the acceptor and donor groups of hydrogen bonds of
atorvastatin calcium and dipicolinic acid molecules. According to the analysis of the PXRD diffractogram, DSC thermogram, and
FTIR spectra, the solid-state of the atorvastatin calcium-dipicolinic acid is a
new multi-component cocrystal (Karagianni, Malamatari, and Kachrimanis, 2018; Pindelska Sokal, and
Kolodziejski, 2017;
Qiao et al., 2011).
3.5. Morphology
The SEM examination aimed to analyze microscopic properties,
including the sample’s surface topography and particle size (Sukeksi et al., 2021; Ali et al.,
2020; Qiao et al., 2011). Figure 5 shows the micrographs of atorvastatin
calcium, dipicolinic acid, and atorvastatin calcium-dipicolinic acid multi-component solid. The atorvastatin calcium powder particles
were rod-shaped, with lengths ranging from 30-100 mm. Also, the surface
topography of drug particles showed surface properties, as stated by the
literature (Wicaksono et al.,
2019). The
dipicolinic acid particles were shaped as a plate light measuring 20-200 mm in length and a flat
surface, as stated in the literature (Hiendrawan
et al., 2016). In addition, the preparation of atorvastatin calcium-dipicolinic acid multi-component solid produced irregularly shaped particles with a
diameter of 15-40 mm and a rough surface. The multi-component
solid showed
microscopic differences with each starting material because the morphological
identity of these materials was not observed in the atorvastatin
calcium-dipicolinic acid multi-component
solid.
Therefore, it can be concluded that the drug and coformer formed a new
multi-component solid system with new physical characteristics and solid-state
properties.
Figure 5 Micrographs
of a) atorvastatin calcium, (b) dipicolinic acid, and (c) atorvastatin calcium-dipicolinic acid
multi-component solid
3.6. Solubility
Solubility is
a parameter that directly affects the bioavailability and pharmacological
effect of an active pharmaceutical ingredient. However, atorvastatin calcium is
an active pharmaceutical ingredient with low solubility. Therefore, developing
the solubility properties of the drug is necessary to prevent the problem of
low bioavailability and lack of pharmacological response when formulated into
pharmaceutical preparations (Anwar,
Warsi, and Mallick, 2011). Table 1 shows the solubility test of pure atorvastatin
calcium and atorvastatin calcium-dipicolinic acid multi-component solid.
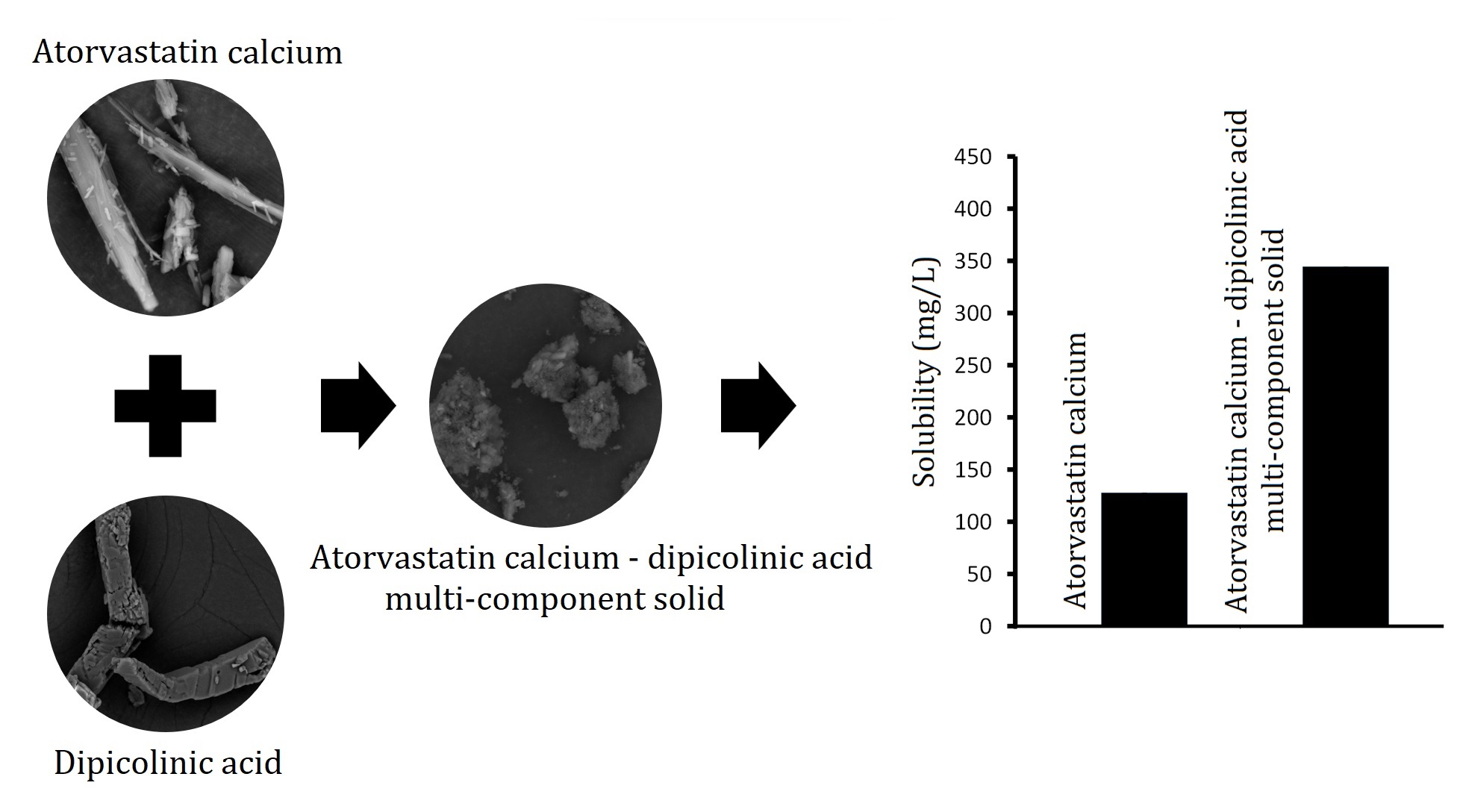
The test showed that the
solubility of atorvastatin calcium was 127.63 ± 7.52 mg/L. This result
indicated that 31.91 mg of atorvastatin calcium would dissolve in 250 water
media. Conversely, atorvastatin calcium is formulated into tablets with doses
of 40 and 80 mg. Therefore, atorvastatin calcium is categorized as a Class II
drug in the biopharmaceutical classification system, indicating low solubility,
and often has bioavailability when formulated into pharmaceutical preparations (Pobudkowska et al., 2015). The solubility of the drug in the multi-component solid was 344.31 ±
37.09 mg/L, which was significantly higher than the pure form (p<0.05).
Therefore, the formation of atorvastatin calcium-dipicolinic acid
multi-component solid can be used to solve the solubility problem of the drug.
Table 1 The solubility of pure
atorvastatin calcium and atorvastatin calcium - dipicolinic acid
multi-component solid in distilled water
|
Replication |
Solubility (mg/L) | |
|
Atorvastatin calcium |
Atorvastatin calcium-dipicolinic acid
multi-component solid | |
|
1 |
121.46 |
385.51 |
|
2 |
136.01 |
333.82 |
|
3 |
125.43 |
313.59 |
|
Mean ± SD |
127.63 ± 7.52 |
344.31 ± 37.09 |
The
solubility of a crystalline solid is influenced by the lattice energies of the
crystal (Elder, Holm, and Diego, 2013). Generally, crystalline solids with lower lattice
energies are more soluble because the constituent molecules are bound in a
lattice with weaker energies (Kuleshova et al., 2013; Thakuria et al., 2013). The
PXRD test showed that the atorvastatin calcium-dipicolinic acid multi-component
cocrystal had a different diffractogram pattern from each constituent
component. Also, the FTIR analysis results showed that the multi-component
solid formation resulted in a new crystalline structure through intermolecular hydrogen
bonds (Khadka et al., 2014). Therefore, atorvastatin calcium-dipicolinic acid
multi-component solid has a higher solubility than the pure form of the drug
due to the decrease in lattice energies of the crystal. This phenomenon caused
the molecules of the drug and coformer to interact more easily with solvent
molecules during the dissolution process (Kuleshova et al.,
2013; Thakuria et al., 2013).
The solvent evaporation-quenching
method was used to convert atorvastatin calcium to a new multi-component solid
with a dipicolinic acid coformer. Furthermore, the PXRD, DSC, FTIR, and SEM
analyses indicated that the solid state of atorvastatin calcium-dipicolinic
acid was a multi-component cocrystal. The solubility testing of the
multi-component solid in distilled water showed a significant improvement of
about 2.7-fold in atorvastatin calcium solubility compared to the pure form.
Therefore, the formation of the multi-component solid can be used to solve the
atorvastatin calcium bioavailability problem due to its low solubility.
Atorvastatin calcium-dipicolinic acid multi-component solid still needs to be
further tested for its in vivo bioavailability so that the multi-component
solid can be formulated into pharmaceutical preparations with better
performance. ?
The authors would like to thank the Rector of the
University of Jember for the research funding through the “Penelitian Pasca Doktor”
(PPD) grant 2020 decree number 15732/UN25/LT/2020 under contract number
3454/UN25.3.1/LT/2020.
Ali, N.H.S.O., Rahman, S.N.S.A., Adol, H.A., Hamid,
M.H.S.A., Taha, H., Rosli, M.Y.H., Kumara, N.T.R.N., Mahadi, A.H., Usman, A.,
2020. Fabrication and Characterization of S-Benzyldithiocarbazate Schiff Base
Microcrystals by a Reprecipitation Method for Enhanced Antibacterial Activity. International
Journal of Technology. Volume 11(7), pp. 1319–1328
Anwar, M., Warsi, M.H., Mallick,
N., 2011. Enhanced Bioavailability of Nano-Sized Chitosan-Atorvastatin
Conjugate after Oral Administration to Rats. European
Journal of Pharmaceutical Sciences, Volume 44, pp.
241–249
Bekbayeva,
L., Negim, E., Niyazbekova , R., Kaliyeva, Z., Yeligbayeva, G., Khatib, J.,
2022. The Effects of Modified Chitosan on the Physicomechanical Properties of
Mortar. International Journal of Technology, Volume 13(1), pp. 125–135
Multicomponent
Crystalline Solid Forms of Aripiprazole Produced via Hot Melt Extrusion
Techniques: An Exploratory Study. Journal of
Drug Delivery Science and Technology, Volume
63, p. 102529
Chadha, R., Sharma, M., Haneef, J., 2017.
Multicomponent Solid Forms of Felodipine: Preparation, Characterisation,
Physicochemical and In-Vivo Studies. Journal
of Pharmacy and Pharmacology, Volume 69, pp. 254–264
Douroumis, D., Ross, S.A., Nokhodchi, A., 2017.
Advanced Methodologies for Cocrystal Synthesis. Advanced Drug Delivery Reviews, Volume 117, pp.
178–195
Elder,
D.P., Holm, R., de Diego, H.L., 2013. Use of Pharmaceutical Salts and Cocrystals to Address the Issue of Poor Solubility. International Journal of Pharmaceutics,
Volume
453, pp. 88–100
Gao,
Y., Gao, J., Liu, Z., Kan, H., Zu, H., Sun, W., Zhang, J., Qian, S., 2012.
Coformer Selection Based on Degradation Pathway of Drugs: A Case Study of
Adefovir Dipivoxil–Saccharin and Adefovir Dipivoxil–Nicotinamide Cocrystals. International Journal of Pharmaceutics.
Volume 438, pp. 327–335
Haneef, J., Chadha, R., 2017. Drug-Drug
Multicomponent Solid Forms: Cocrystal, Coamorphous and Eutectic of Three Poorly
Soluble Antihypertensive Drugs Using Mechanochemical Approach. AAPS PharmSciTech, Volume 18(6), pp.
2279–2290
Hiendrawan, S., Veriansyah, B.,
Widjojokusumo, E., Soewandhi, S.N., Wikarsa, S., Tjandrawinata, R.R., 2016.
Simultaneous Cocrystallization and Micronization of Paracetamol-Dipicolinic
Acid Cocrystal by Supercritical Antisolvent (SAS). International Journal of
Pharmacy and Pharmaceutical Sciences, Volume 8(2), pp.
89–98
Javeer,
S.D., Patole, R., Amin, P., 2013. Enhanced Solubility and Dissolution of
Simvastatin by HPMC-Based Solid Dispersions Prepared by Hot Melt Extrusion and
Spray-Drying Method. Journal of
Pharmaceutical Investigation, Volume 43, pp. 471–480
Karagianni, A., Malamatari, M.,
Kachrimanis, K.,
2018. Pharmaceutical Cocrystals: New Solid Phase
Modification Approaches for the Formulation of APIs. Pharmaceutics,
Volume
10(18), pp. 1–30
Kawabata, Y., Wada, K., Nakatani, M., Yamada, S., Onoue, S., 2011. Formulation
Design for Poorly Water-Soluble Drugs Based on Biopharmaceutics Classification
System: Basic Approaches and Practical Applications. International Journal of Pharmaceutics, Volume 420, pp. 1–10
Khadka, P., Ro, J., Kim, H., Kim,
L. Kim, J.T., Kim, H., Cho, J.M., Yun, G., Lee, J., 2014. Pharmaceutical
Particle Technologies: An Approach to Improve Drug Solubility, Dissolution, and
Biavailibility. Asian Journal of Pharmacy and Pharmaceutical Sciences, Volume
9(6), pp. 304–316
Kuleshova, L.N., Hofmann, D.W.M.,
Boese, R., 2013. Lattice Energy Calculation- A Quick Tool for Screening of
Cocrystal and Estimation of Relative Solubility. Case of Flavonoids. Chemical
Physics Letters, Volume 564,
pp. 26–32
Maggi,
L., Canobbio, A., Bruni, G. Musitelli, G., Conte, U., 2015. Improvement of the
Dissolution Behavior of Gliclazide, a Slightly Soluble Drug, Using Solid
Dispersions. Journal of Drug Delivery
Science and Technology, Volume 26, pp. 17–23
Pindelska, E., Sokal, A., Kolodziejski, W.,
2017. Pharmaceutical Cocrystals, Salts and Polymorphs: Advanced
Characterization Techniques. Advanced
Drug Delivery Reviews, Volume 117, pp. 111–146
Pobudkowska,
A., Domanska, U., Jurkowska, B.A., Dymczuk, K., 2015. Solubility of
Pharmaceuticals in Water and Alcohols. Fluid
Phase Equilibria, Volume 392, pp. 56–64
Qiao, N., Li, M., Schlindwein, W., Malek, N.,
Davies, A., Trappitt, G., 2011. Pharmaceutical Cocrystals: An Overview. International Journal of Pharmaceutics,
Volume 419, pp. 1– 11
Shaikh, R., Singh, R., Walker,
G.M., Croker, D.M., 2018. Pharmaceutical Cocrystal Drug Products: An Outlook on
Product Development. Trends in
Pharmacological Sciences, Volume 39(12), pp.
1033–1048
Shete, G., Puri, V., Kumar, L., Bansal,
A.K., 2010. Solid State Characterization of Commercial Crystalline and
Amorphous Atorvastatin Calcium Samples. AAPS PharmSciTech, Volume
11(2), pp. 598–609
Sukeksi, L.,
Iriany, Grace, M., Diana, V., 2021. Characterization of the Chemical and Physical
Properties of Bar Soap Made with Different Concentrations of Bentonite as a
Filler. International
Journal of Technology, Volume 12(2), pp. 263–274
Thakuria, R., Delori, A., Jones, W., Lipert,
M.P., Roy, L., Hornedo, N.R., 2013. Pharmaceutical Cocrystals and Poorly
Soluble Drugs. International Journal of
Pharmaceutics, Volume 453, pp. 101–125
Tilborg, A., Michaux, C.,
Norberg, B., Wouters, J., 2010. Advantages of Cocrystallization in the Field of
Solid-State Pharmaceutical Chemistry: L-Proline and MnCl2. European Journal of Medicinal Chemistry,
Volume
45, pp. 3511–3517
Wicaksono, Y., Rosidi, V.A., Saragih, S.Y., Fauziah,
L.S., Setyawan, D., 2020. Preparation
of Spray Dried Coamorphous Solids to Improve the Solubility and Dissolution
Rate of Atorvastatin Calcium. Jurnal
Teknologi, Volume 83(2),
pp. 77–83
Wicaksono, Y., Setyawan, D.,
Siswandono, S., 2018. Multicomponent
Crystallization of Ketoprofen-Nicotinamide for Improving the Solubility and
Dissolution Rate. Chemistry Journal of Moldova, Volume 13(2), pp. 74–81
Wicaksono, Y., Setyawan, D.,
Siswandono, S., Siswoyo, T.A. 2019. Preparation and
Characterization of a Novel Cocrystal of Atorvastatin Calcium with Succinic
Acid Coformer. Indonesian Journal
of Chemistry, Volume 19(3), pp.
660 – 667
Williams, H.D., Trevaskis, N.L., Charman, S.A., Shanker,
R.M., Charman, W.N., Pouton, C.W., Porter, C.J.H., 2013. Strategies to Address
Low Drug Solubility in Discovery and Development. Pharmacological Reviews, Volume 65, pp. 315–499
Zaini, E., Afriyani, A., Fitriani, L., Ismed, F.,
Horikawa, A., Uekusa, H., 2020. Improved Solubility and Dissolution Rates in
Novel Multicomponent Crystals of Piperine with Succinic Acid. Scientia Pharmaceutica, Volume
88(21), pp. 1–12
