Nanofiber Cellulose/Lignin from Oil Palm Empty Fruit Bunches and the Potential for Carbon Fiber Precursor Prepared by Wet-spinning
Published at : 18 Jan 2023
Volume : IJtech
Vol 14, No 1 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i1.5082
Harahap, M., Daulay, N., Zebua, D., Gea, S., 2023. Nanofiber Cellulose/Lignin from Oil Palm Empty Fruit Bunches and the Potential for Carbon Fiber Precursor Prepared by Wet-spinning. International Journal of Technology. Volume 14(1), pp. 152-161
| Mahyuni Harahap | 1. Department of Chemistry, Faculty of Science Technology and Information, Universitas Sari Mutiara Indonesia, Jalan Kapten Muslim, Medan 20124, Indonesia. 2. Cellulosic and Functional Materials Rese |
| Nurkholidah Daulay | Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Sumatera Utara, Jalan Bioteknologi No.1, Medan 201555, Indonesia |
| Devi Zebua | Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Sumatera Utara, Jalan Bioteknologi No.1, Medan 201555, Indonesia |
| Saharman Gea | 1. Cellulosic and Functional Materials Research Centre, Universitas Sumatera Utara, Jalan Bioteknologi No.1, Medan 20155, Indonesia. 2. Department of Chemistry, Faculty of Mathematics and Natural Sci |
This work isolated nanofiber cellulose (NFC) and Lignin from oil palm
empty fruit bunches (OPEFB). NFC/lignin-based carbon fiber was prepared by
using the wet-spinning technique and followed by pyrolysis at 500°C. Lignin isolated from OPEFB was
compared with commercial Lignin by using Fourier Transform Infrared (FTIR). The
nano dimension of NFC was observed by using Transmission Electron Microscope
(TEM). The NFC/lignin after pyrolysis was characterized by using SEM EDX and
Raman spectroscopy. NFC showed diameters between 5 nm and 60 nm. Both Lignin
commercial and isolated had aromatic benzene. Lignin was introduced to NFC,
where NFC/lignin composition of (100:0), (90:10), and (80:20) increased the
thermal stability of wet-spun fiber. Meanwhile, NFC/lignin (90:10) and (80:20)
had almost 50% residual mass. The result confirmed that the carbon material,
such as NFC/lignin (100:0), (90:10), and (80:20), had rod-like shape morphology
with diameters of 236.2 nm, 492.4 nm, and 653.2 nm, respectively. Furthermore,
the G band and D band of the carbonous material were observed at 1580 cm-1 and 1358 cm-1. The conversion of NFC/lignin
from OPEFB to carbon material was successful and potentially suitable for the
precursor of carbon fiber.
Carbon fiber; Lignin; Nanofiber cellulose; Oil palm empty fruit bunches
Carbon fiber is a
great research interest because of its large surface area, high porosity, low
density, sufficient electrical conductivity, and lightweight. Carbon fiber
demand was reported to increase by 10% annually (Wang et
al., 2019), as it
has become an important engineering material due to its unique and attractive
mechanical properties (stronger than steel and aluminum). Carbon fiber
reinforces polymer-matrix composites in advanced materials.
Polyacrylonitrile (PAN) is the most
suitable precursor for carbon-based material, accounting for high-performance
carbon fiber, high melting point, and rich carbon content. However, PAN is
petroleum-based with complex and costly manufacturing processes of the
functional materials, whose cost could be twice the carbon processing cost (Baker and Rials, 2013).
Furthermore, the increase in environmental awareness has sparked an interest in
materials produced from natural resources. Therefore, green carbon source as an
alternative to replacing petroleum-based polymers is more favored in
manufacturing carbon fiber.
Cellulose, the most abundant biomass resource,
originated from plants and microorganisms such as algae and tunicate. The
utilization of cellulose reduced the dependence on fossil resources and
protected the environment. Cellulose is environmentally friendly, abundantly
available, renewable, biodegradable, and biocompatible. However, the use of
cellulose as the precursor for carbon fiber has some limitations, such as
relatively low carbon content and aromatic absence in the biopolymers.
Nanofiber cellulose (NFC), highly crystalline cellulose, has been used to
improve the properties of material cellulose-based, i.e., nanofiber
composite (Misran et
al., 2020), printed electronics (Hoeng, Denneulin, and Bras, 2016), biomedicine (Lin and Dufresne, 2014), nanocomposite (Helmiyati and
Anggraini, 2019), nanofiltration membrane (Rochardjo et
al., 2021), and filler for carbon fiber (Harahap et
al. 2019).
Lignin, the second most abundant biomass in the world, is a major
potential source of renewable aromatic resources (Ház et al.,
2019). It consists of three main phenylpropane
units, such as guaiacol (G), sryingyl (S), and p-hydroxyphenyl (H), at
different proportions depending on the sources and extraction process. In the
pulp industry, only 1%-2% of Lignin produced could be utilized, while the rest
was usually discharged directly to the sewage which could cause serious
environmental pollution (Baker and
Rials, 2013). A review of “global lignin supply overview
and kraft lignin potential as an alternative to petroleum-based polymer” stated
that kraft lignin production increased by 150% between 2014 and 2018, and
market pull for Lignin is projected between 2023 and 2028. Lignin has been a
proven alternative to petroleum energy, mainly due to its availability at
around one-third of the solid matter in woods (Dessbesell et
al., 2020). Preparing a high-value lignin-based
material remains a prominent issue in order to utilize this natural biopolymer
fully and reduce the contamination from pulp industry, considering low
mechanical properties of Lignin where tensile modulus, strength, and
strain-to-failure-values-were-accounted-for 52 ± 2 GPa, 1.04 ± 0.10 GPa, and
2.0 ± 0.2%, respectively (Zhang and Amod, 2014).
Several studies have reported the potential of lignin
application in advanced materials by combining Lignin with other polymers or
modifying it. Lignin-polyvinyl alcohol nanofiber was formulated for
microelectrode applications (Roman et
al., 2019). Other literature converted
lignin-polyacrylonitrile to carbon nanofiber (Ma, Li,, and
Chang, 2015). Lignin was also acetylated to ?enhance its functionality and exploit its application areas (Zhao et
al., 2017). Wang et al.
(2019) isolated nanocellulose from pulp hardwood and spun it with
commercial Lignin to produce conductive carbon microfibers. In addition, Geng et al. (2021) produced a highly strong
and conductive carbon fiber based-nanocellulose from jute fiber and sodium
lignosulfonate crosslinked by chitosan molecule.
In this study, we aim to prepare
biodegradable and green carbon fiber by using NFC and Lignin from oil palm
empty fruit bunches (OPEFB). Our previous study reported a simple method to
extract NFC and Lignin from OPEFB without producing by-product (Gea
et al., 2020). To the best of our
knowledge, there is no study yet reported carbon fiber-based NFC and Lignin
derived from OPEFB. ?We expect that blending NFC
and Lignin would increase the mechanical properties of lignin-based carbon
fiber. Furthermore, the production cost of NFC/lignin-based carbon fiber is
estimated to be lower than petroleum-based carbon fiber.
OPEFB was obtained
from PTPN IV Adolina, North Sumatra, Indonesia. The materials used in this
experimental were HNO3, NaNO2, NaOH pellets (>98%),
NaSO3, NaOCl (2.5%), H2O2 (30%), HCl (37%), H2SO4
(98%), DMSO (98%) purchased from Merck (Darmstadt, Germany). All materials were
used without further purification.
2.1. Isolation of Nanofiber Cellulose and Lignin
from Oil Palm Empty Fruit Bunches
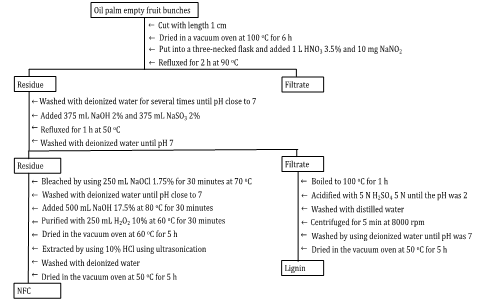
OPEFB was cut into small
pieces with lengths around 1 cm. They were dried in a vacuum oven at 100![]() for six h. Next, 75 g of dried
OPEFB was put into a three-necked flask where 1 L HNO3 3.5% and 10
mg NaNO2 were added. The mixture was refluxed for two h at 90°C. The residue was collected and washed with
deionized water several times until pH was close to 7. After that, 375 mL NaOH
2% and 375 mL NaSO3 2% were added, and the reflux was continued for
one h at 50°C. Next, the
residue was collected and washed with deionized water until pH 7, while the
filtrate was collected for the lignin isolation process.
for six h. Next, 75 g of dried
OPEFB was put into a three-necked flask where 1 L HNO3 3.5% and 10
mg NaNO2 were added. The mixture was refluxed for two h at 90°C. The residue was collected and washed with
deionized water several times until pH was close to 7. After that, 375 mL NaOH
2% and 375 mL NaSO3 2% were added, and the reflux was continued for
one h at 50°C. Next, the
residue was collected and washed with deionized water until pH 7, while the
filtrate was collected for the lignin isolation process.
Next, the filtrate collected for lignin isolation was boiled at 100°C for one h. It was then acidified with 5 N H2SO4 5 N until the pH was 2. Next, it was washed with distilled water and centrifuged for 5 min at 8000 rpm at room temperature. Then, it was washed by using deionized water until pH was 7. The Lignin was dried in the vacuum oven at 50°C for five h. The scheme illustration for NFC and lignin isolation is shown in Figure 1.
Figure 1 A scheme
illustration for NFC and lignin isolation from OPEFB
2.2. Wet-spinning of Nanofiber
NFC was dissolved in DMSO under reflux
conditions at 60°C for 12 h with a 10% (w/v) concentration. The solution was
allowed to cool at room temperature. The same condition was also carried out
for the lignin solution. After that, three polymer solutions (NFC/lignin) with
the concentration of (100:0); (90:10); and (80:20) were dispersed by sonication
at 24 kHz, 20°C –30°C for 10 minutes. The solutions were spun in a coagulation
bath that contained a deionized water solution. The wet-spun condition was as
follows: needle diameter of 0.25 mm, feed rate of 0.004 g/minute, and wind
speed of 0.44 m/minute. The spun fiber was dried at room temperature and coded
as NFC/lignin (100:0); NFC/lignin (90:10); and NFC/lignin (80:20).
2.3.
Carbonization of Wet-spun Fiber
Carbonization of the wet-spun NFC/lignin
fibers was performed by using a tube furnace under a nitrogen atmosphere (200
mL/minute). First, 3 g of the sample was placed in a graphite sample holder and
placed inside the tube furnace. Then, pyrolysis with a heating rate of 5°C /minute
was done in two steps: pre-carbonization from 25°C to 100°C, held isothermally
for 30 minutes at 100°C, and carbonization between 100°C and 500°C, held
isothermally for 30 minutes at 500°C.
2.4.
Characterization
First, the NFC dimension was investigated
using JEOL/EO JEM-1400 (Tokyo, Japan) transmission electron microscope operated
at an accelerating voltage of 100 kV. A highly diluted NFC solution in
distilled water was prepared. The solution was dropped on carbon-coated
electron microscope grids and stained with uranyl acetate. Next, the morphology
of Lignin and NFC/lignin fiber was characterized by using Hitachi TM3030 (JEOL,
Ltd., Tokyo, Japan) scanning electron microscope (SEM). The sample was first
coated with a thin layer of gold to reduce charges during analysis. The
instrument was operated at an accelerating voltage of 10 kV. Third, the
solution's chemical compounds were studied using a Fourier-transform infrared
(FTIR) spectrometer (Nicolet 380, Thermo Scientific, Boston, USA), which used a
transmission mode with a resolution of 4 cm-1 and 100 scans. Fourth,
the crystallinity of NFC, Lignin, and NFC/lignin fibers was investigated by
using Bruker D8 advanced X-ray diffractometer (Bruker Optik GmbH, Ettlingen,
Germany). The instrument was operated at 35 kV and 25 mA. Next, the samples'
thermal analysis was carried out using thermogravimetric analysis (TGA) and
DTA/TG Exstar SII 7300 (Hitachi medical system, Tokyo, Japan), where 10 mg
sample was placed in a platinum pan and tightly closed. The sample was analyzed
between 30°C and 600°C at a heating rate of 10°C/min. Finally, the morphology
of the carbonized NFC/lignin was observed by using SEM EDX, while the structure
was investigated by using Raman spectroscopy (Raman microscope enclosure,
RE-04, Renishaw, UK) using a 785 nm laser. The samples were analyzed without
any treatment. The analyzed region was visualized through a microscope with 50x
magnification. The range of wavelengths collected was typically from 150 to
3200 cm-1 over a time period of at least 30 seconds and multiple
accumulations for high sensitivity.
3.1. FTIR
Analysis
FTIR spectra for NFC and Lignin isolated from
OPEFB, commercial Lignin, wet-spun NFC/lignin are shown in Figure 2. Both
isolated and commercial Lignin had OH groups stretching in phenolic and
aliphatic structures at wavenumber 3430 cm-1. C-H stretching in
methyl and methylene groups and methoxy groups was shown at the absorption
bands of 2925 cm-1 and 2850 cm-1, respectively. Two
string vibrations were observed at 1515 cm-1 and 1637 cm-1
corresponding to the aromatic benzene ring. Syringyl units in Lignin were seen
at 1328 cm-1, 1228 cm-1, and 1125 cm-1 bands,
whereas guaiacyl structures appeared at 1272 cm-1 and 1032 cm-1
bands (Arun et al., 2020). The band at 1168 cm-1 corresponded to
the carbonyl stretching of conjugated ester groups. Similarly, the band from
1000 cm-1 to 800 cm-1 attributed to C-O, C-H, and C=O
aromatic out-of-the-plane deformation in Lignin (Arun
et al., 2020).
NFC/lignin spun-fiber showed absorption bands between 4000 and 3200 cm-1 corresponding to the hydroxyl groups (-OH) of water. This trend has become larger compared to isolated and commercial Lignin. In addition, C-O of the alcohol group in NFC could be clearly seen in NFC/lignin spun-fiber at the 1087 cm-1 to 1026 cm-1 band. For NFC, O-H and H-C-H stretching (alkyl, aliphatic) were seen at 3278 cm-1 and 2890 cm-1, respectively. The band at 1640 cm-1 corresponded to fiber-OH (absorbed water), 1429 cm-1 attributed to HCH and OCH bending vibrations (methylene group), and 1371 cm-1 referred to CH deformation vibration (CH3 or OH in plane). The band confirmed COC, CCO, and CCH deformation and stretching vibrations at 896-915 cm-1 (Xie et al., 2016).
Figure 2 FTIR spectra of NFC, isolated Lignin, commercial Lignin, NFC/lignin
(100:0), NFC/lignin (90:10), and NFC/lignin (80:20)
3.2. Morphological
Analysis
The dimension of NFC was
analyzed using TEM, while the morphological analysis of Lignin and wet-spun NFC/lignin
was carried out using SEM. TEM micrograph of NFC and SEM images of Lignin and
wet-spun NFC/lignin are shown in Figures 3 and 4, respectively.
Figure 3 TEM micrograph of NFC isolated from OPEFB with (a) 100 nm and (b) 200 nm
scale bars
Figure 4 SEM micrographs of: (a) lignin, (b) NFC/lignin
(100:0), (c) NFC/lignin (90:10), and (d) NFC/lignin (80:20) with 1.0 K
magnification
With reference to Figure 2,
NFC showed long fibers and web-like shapes with diameters between 5 nm and 60
nm. The same result was reported by another study claiming that NFC from
pineapple peels isolated by the steam explosion had a diameter in size range of
5–60 nm (Cherian et al., 2010). On the other hand, the dimension of NFC isolated
from Ushar (Calotropid Procea) was in size range of 10–20 nm (Oun and Rhim, 2016), whereas the NFC from cotton linter was in the size
of 12 nm (Morais et al., 2013). The shape and dimension of NFC were commonly
affected by the source of cellulose, isolation method, and reaction
condition.
The SEM images of Lignin
isolated from OPEFB (Figure 3.a) had a uniform, flake-like, rough surface
morphology. However, SEM images of NFC/lignin 100:0, 90:10, and 80:20 (Figure
3.b, 3.c, and 3.d) showed non-uniform morphology. They looked like single
fibers but attached to each other. Pores were observed on those fibers, where
the higher the lignin content, the bigger the pore sizes were. NFC/lignin
(80:20) showed particles on the fiber surface that were not observed in
NFC/lignin (100:0) and (90:10). This appearance may be due to the sonication
time of NFC/lignin that was being too short. Higher lignin content should be
sonicated longer with more lignin particles dispersed into NFC.
3.3. Thermogravimetric
Analysis
The thermal stability of Lignin, NFC, and NFC/lignin (90:10, and 80:20) was analyzed using thermogravimetric analysis (TGA). The TGA traces for all samples are presented in Figure 5. All samples had a three-stage processing time function indicating sample weight loss.
Figure 5 TGA curve for Lignin, NFC, NFC/lignin (100:0), NFC/lignin (90:10), and
NFC/lignin (80:20)
3.4. XRD
Analysis
XRD analysis was carried out to confirm the crystallinity of NFC before and after blending with Lignin. Figure 6 shows the XRD pattern for Lignin, NFC, and wet-spun NFC/lignin (90:10). Lignin had an 18.30o-18.40o reflection attributed to the amorphous phase. At the same time, NFC had 22.3o reflection assigned to the crystallinity of NFC. This peak was high owing to NFC being a high crystalline material, with crystallinity >70% (Gea et al., 2020). From the pattern, NFC had the structure of cellulose I with two theta reflection at 14.5o – 15.3o, 15.7o–16.30o, and 21.90o–22.20o attributed to (1-10), (100), and (200) crystallographic plane respectively. The presence of Lignin in the NFC did not disturb the crystallinity.
Figure 6 XRD pattern of lignin, NFC, and NFC/lignin (90:10)
3.5. Characterization of Carbon
Fibers
3.5.1. SEM EDX Analysis
The morphology of wet-spun NFC/lignin after carbonization was investigated by using SEM EDX, as shown in Figure 7. Carbon fiber from NFC/lignin (100:0) Figure 7(a) shows the rod-like shape morphology. This sample had the smoothest surface morphology compared to the other fibers. The morphology of NFC/lignin (90:10) Figure 7(b) also shows a rod-like shape, but the fibers were stacked at each other. On the other hand, NFC/lignin (80:20) Figure 7(c) had rod-like and spherical morphology. The diameters were directly measured by using an SEM EDX machine, providing 236.2 nm, 492.4 nm, and 653.2 nm measurements for carbon fiber NFC/lignin (100:0), (90:10), and (80:20), respectively. All samples had approximately 50% oxygen and >25% sodium and carbon content. NFC/lignin (90:10) and NFC/lignin (80:20) contained 0.06% sulfur and 0.05% silicon. Another study reported that carbon fiber derived from Lignin and NFC pyrolyzed at 1000°C had >80% carbon content (Geng et al., 2020). Higher temperatures would increase the carbon content and decrease the oxygen content.
Figure 7 SEM images of wet-spun NFC/lignin (100:0), NFC/lignin (90:10), and
NFC/lignin (80:20) carbon fibers
3.5.2. Raman Analysis
Raman spectroscopy studied
the carbon structure of wet-spun NFC/lignin (100:0; 90:10; and 80:20). The
Raman spectra for the carbon fiber are presented in Figure 8. The crystalline
structure of carbon material in Raman spectroscopy showed four spectral regions
to indicate either amorphous or the crystalline parts in carbon as summarized
in the following: D (1350-1370 cm-1), G (1575-1582 cm-1),
D’ (~1620 cm-1) and G’ (2690-2730 cm-1). G-band was for
sp2 bonding of C=C bonds in the graphitic structures with E2g
vibration mode. It is normally presented in a single graphite crystal. Whereas,
D-band represented sp3 bonding in poorly graphitized fibers.
Therefore, according to the Tuinstra-Koenig equation, the ID/IG ratio could
determine the order level of carbon materials. The peak at ~1620 cm-1
appeared for non-graphitized fibers; whereas the band at 2690-2730 cm-1
presented graphitized fibers (Le et al. 2020).
From Figure 7, the peaks for G band and D band were at 1580 cm-1 and 1358 cm-1, indicating the presence of carbon material in the wet-spun fiber NFC/lignin (100:0; 90:10; and 80:20) with ID/IG values of 0.84, 0.86, and 0.84 respectively. The results provided information that the carbonization process for all wet-spun fiber was successful, and the fiber had a potential use for carbon fiber precursor. However, in the next study, the pyrolysis temperature could be increased to 1000°C in order to obtain higher G band. Table 1 summarises several applications of carbon fiber derived from biomass.
Figure 8 Raman spectra of NFC/lignin (100:0), NFC/lignin (90:10), and NFC/lignin
(80:20) carbon fibers
Table 1 Carbon fiber derived from biomass
|
Raw material |
Method |
Application |
References |
|
Bamboo |
Lay-up method |
Composite |
(Pramono,
Rebet, and Zulfa, 2017) |
|
Nanocellulose from jute fibre/sodium lignosulfonate |
Wet-spinning
|
Conductive carbon fibre |
(Geng et al., 2021) |
|
Nanocellulose/lignin |
Wet-spinning |
Conductive carbon fibre |
(Wang et al., 2019) |
NFC-isolated OPEFB have diameters between
5 nm and 60 nm with long fibers and web-like shapes, whereas Lignin has
uniform, flake-like, and rough surface morphology. The introduction of Lignin
into NFC nanofiber increases the thermal stability and residual mass and maintains the
crystallinity. Carbon fiber from wet-spun NFC/lignin carbonized at 500°C has
rod-like shape morphology. NFC/lignin (100:0) has the smoothest surface
morphology compared to other carbon fibers. The diameter of the carbon fiber
increases with the presence of Lignin into NFC, where diameters of 236.2 nm,
492.4 nm, to 653.2 nm have resulted in NFC/lignin (100:0), (90:10), (80:20)
samples respectively. The carbon content of all samples is >25%. Raman
spectroscopy confirms the G band (1580 cm-1) and D band (1358 cm-1)
for all carbonous NFC/lignin indicating the presence of carbon material in the
samples. The carbonization process for all wet-spun NFC/lignin fiber is
successful, and the fiber can be used as a carbon fiber precursor.
This research received no specific grant from any
funding agencies in the public, commercial, or not-for-profit sectors.
Arun, V., Perumal, E.M., Prakash, K.A.,
Rajesh, M., Tamilarasan, K., 2020. Sequential fractionation and characterization
of lignin and cellulose fiber from waste rice bran. Journal of Environmental
Chemical Engineering, Volume 8(5), pp. 1–19
Baker, D.A., Rials, T.G., 2013. Recent advances
in low-cost carbon fiber manufacture from lignin. Journal of Applied Polymer
Science, Voulme 130(2), pp. 713–28
Cherian, B.M., Leao, A.L., Souza, S.F.d.,
Thomas, S., Pothan, L.A., Kottaisamy, M., 2010. Isolation of nanocellulose from
pineapple leaf fibres by steam explosion. Carbohydrate Polymers, Volume
81(3), pp. 720–25
Dessbesell, L., Paleologou, M., Leitch, M.,
Pulkki, R., Xu, C.C., 2020. Global lignin supply overview and kraft lignin
potential as an alternative for petroleum-based polymers. Renewable and
Sustainable Energy Reviews, Volumer 123, pp. 109768
Gea, S., Siregar, A.H., Zaidar, E.,
Harahap, M., Indrawan, D.P., Perangin-angin, Y.A., 2020. Isolation and characterisation
of cellulose nanofibre and lignin from oil palm empty fruit bunches. Materials,
Volume 13(10), pp. 2290
Geng, L., Cai, Y., Lu, L., Zhang, Y., Li,
Y., Chen, B., Peng, X., 2021. Highly strong and conductive carbon fibers
origanted from bioinspired lignin/nanocellulose precursor obtained by
flow-assited alignmnet and in situ interfacial complexation. ACS Sustainable
Chemistry & Engineering. Volume 9(6), pp. 2591–2599
Geng, S., Wei, J., Jonasson, S., Hedlund,
J., Oksman, K., 2020. Multifunctional carbon aerogels with hierarchical
anisotropic structure derived from lignin and cellulose nanofibers for CO2
capture and energy storage. ACS
Applied Materials and Interfaces, Volume 12(6), pp. 7432–41
Harahap, M., Hararak, B., Khan, I.,
Pandita, S., Gea, S., 2019. Wet-spinning of cellulose acetate reinforced with
acetylated nano-crystalline cellulose as carbon fibre precursors. IOP
Conference Series: Materials Science and Engineering, Volumee 553, pp. 1–8
Ház, A., Jablonsky, M., Surina, I., Kacik,
F., Bubenikova, T., Durkovic, J., 2019. Chemical composition and thermal
behavior of kraft lignins. Forests, Volume 10(6), pp. 1–12
Helmiyati, Anggraini,
Y., 2019. Nanocomposites comprising cellulose and nanomagnetite as
heterogeneous catalysts for the synthesis of biodiesel from oleic acid. International
Journal of Technology, Volume 10(4), pp. 808–17
Hoeng, F., Denneulin, A., Bras, J., 2016.
Use of nanocellulose in printed electronics: a review. Nanoscale, Volume
8(27), pp. 13131–54
Le, N.D., Trogen, M., Ma, Y., Varley, R.J.,
Hummel, M., Byrne, N., 2020. Cellulose-lignin composite fibers as precursors
for carbon fibers: part 2–the impact of precursor properties on carbon fibers. Carbohydrate
Polymers, Volume 250, pp. 116918
Lin, N., Dufresne, A., 2014. Nanocellulose
in biomedicine: current status and future prospect. European Polymer
Journal, Volume 59, pp. 302–25
Ma, A., Li. Z., Chang, J, 2015. Conversion
of lignin-nanofibers to CNFs. Nano, Volume 10(6), pp. 1–9
Misran, E., Wirdjosentono, B., Noor, N.M.,
Gea, S., Situmorang, S.A., Harahap, M., 2020. Preparation and characterisation
of electrospun composite nanofibre polyvinyl alcohol/nanofibrillated cellulose
isolated from oil palm empty fruit bunches. BioResources, Volume 15(4),
pp. 7906–17
Morais, J.P.S., Rosa, M.d.F., Filho,
M.M.S., Nascimento, L.D., Nascimento, D.M., Cassales, A.R., 2013. Extraction
and characterization of nanocellulose structures from raw cotton linter. Carbohydrate
Polymers, Volume 91(1), pp. 229–35
Oun, A.A., Rhim, J.W., 2016.
Characterization of nanocelluloses isolated from ushar (calotropis procera)
seed fiber: effect of isolation method. Materials Letters, Volume 168,
pp. 146–50
Pramono, A.E., Rebet, I., Zulfa, A., 2017.
Electrical and mechanical properties of phenolic resin and gigantochloa apus
carbon fibre composite. International Journal of Technology, Volume 5,
pp. 920–929
Rochardjo, H.S.B., Fatkhurrohman., Ahmad,
K., Yudhanto, F., 2021. Fabrication of nanofiltration membrane based on
polyvinyl alcohol nanofibers reinforced with cellulose nanocrystal using
electrospinning techniques. International Journal of Technology, Volume
12(2), pp. 329
Roman, J., Neri, W., Derré, A., Poulin, P.,
2019. Electrospun lignin-based twisted carbon nanofibers for potential
microelectrodes applications. Carbon, Volume 145, pp. 556–64
Wang, L., Ago, M., Borghei, M., Ishaq, A.,
Papageorgiou, A.C.P., Lundahl, M., Rojas, O.J., 2019. Conductive carbon
microfibers derived from wet-spun lignin/nanocellulose hydrogels. ACS
Sustainable Chemistry and Engineering, Volume 7(6), pp. 6013–22
Xia, Z., Li, J., Zhang, J., Zhang, X.,
Zheng, X., Zhang, J., 2020. Processing and valorization of cellulose, lignin
and lignocellulose using ionic liquids. Journal
of Bioresources and Bioproducts, Volume 5, pp. 79–98
Xie, J., Hse, C.H., Hoop, C.F.C., Hu, T.,
Qi, J., Shupe, T.F., 2016. Isolation and characterization of cellulose nanofibers
from bamboo using microwave liquefaction combined with chemical treatment and
ultrasonication. Carbohydrate Polymers, Volume 151, pp. 725–34
Zhang, M., Amod A.O., 2014. Carbon fibers
from dry-spinning of acetylated softwood kraft lignin. Carbon, Volume
69, pp. 626–29
Zhao, X., Huang, Z., Zhang, Y., Yang, M.,
Chen, D., Huang, K., Hu, H., Huang, A., Qin, X., Feng, Z., 2017. Efficient solid-phase
synthesis of acetylated lignin and a comparison of the properties of different
modified lignins. Journal of Applied
Polymer Science, Volume 134(1), pp. 1–13
