Silica-Chitosan Nanocomposite Coatings for Enhancing Hydrophilicity of Polyester Fabric
Corresponding email: nuruddin@tf.itb.ac.id
Published at : 28 Jun 2023
Volume : IJtech
Vol 14, No 4 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i4.4786
Mulyani, R.W.E., Nuruddin, A., Suprijanto, Sunendar Purwasasmita, B., 2023. Silica-Chitosan Nanocomposite Coatings for Enhancing Hydrophilicity of Polyester Fabric. International Journal of Technology. Volume 14(4), pp. 761-769
| Rr. Wiwiek Eka Mulyani | 1. Doctoral Program of Engineering Physics, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132, Indonesia, 2. Textile Chemistry Department, Polytechnic of Textile Technology, |
| Ahmad Nuruddin | Advanced Functional Materials Research Group, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132, Indonesia |
| Suprijanto | Instrumentation and Control Research Group, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132, Indonesia |
| Bambang Sunendar Purwasasmita | Laboratory of Advanced Materials Processing, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132, Indonesia |
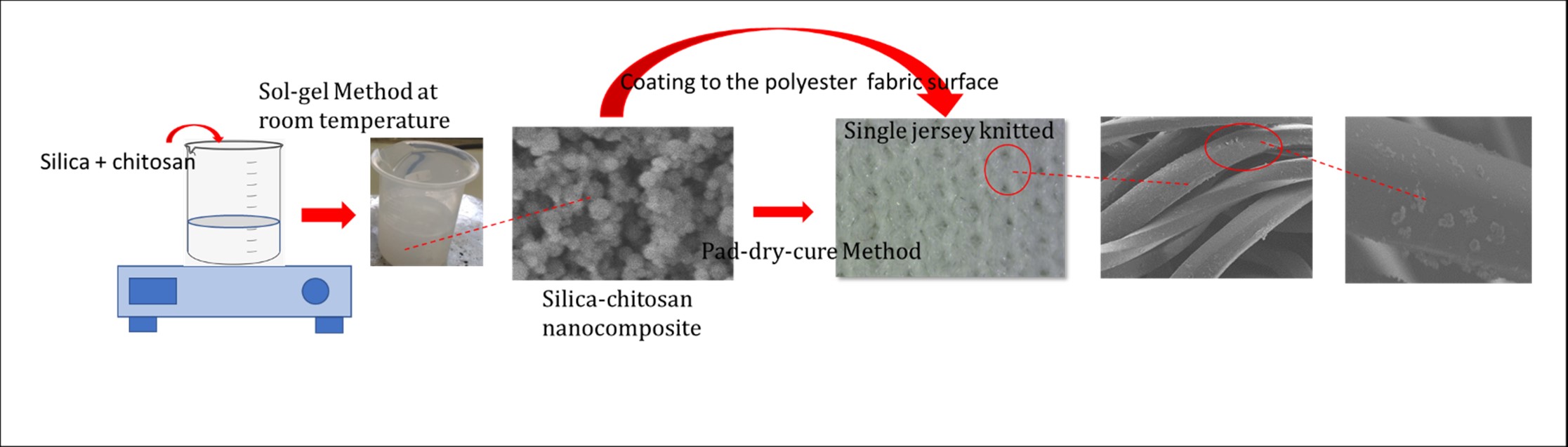
The hydrophilicity of polyester fabric surfaces has
been modified using silica-chitosan nanocomposites. The silica-chitosan
nanocomposite was synthesized by the sol-gel method from sodium silicate and
various chitosan concentrations of 0 – 1.5% at a pH of 3 – 5. A single jersey
knitted polyester fabric was coated by silica-chitosan nanocomposite using the
pad-dry-cure method. It was found that the chitosan concentration and the
solution pH controlled the formation of various size distributions of sphere
nanocomposites with an average size of 96.0-201nm. The coated polyester fabric
with sphere silica-chitosan exhibits a rough surface and produces a contact
angle approaching 0°, facilitating the polyester fabric's speed-up water
absorption and hydrophilic properties.
Chitosan; Hydrophilic; Nanocomposite; Polyester; Sodium silicate
Synthetic
fibers such as polyester are the most popular and common fiber used in
activewear and sportswear. It is cheap, easy to manufacture, and has excellent
washing and wearing properties (Shishoo,
2005). Polyester
fibers also have a good wicking rate and better dimensional stability (Ramakrishnan
and Jagannathan, 2018). However,
the fiber is generally hydrophobic and has a much lower water absorption
capacity. Polyester deficiencies are usually overcome by improving the wicking
ability through chemical treatment, which is achieved by applying a hydrophilic
coating to each polyester filament. The resulting hydrophilic surface allows
moisture to migrate along the outer surface of the filament.
Researchers have tried to modify the polyester surface's hydrophilicity, for example, by blending polyester with cellulosic (Zaman et al., 2013; Troynikov and Wardiningsih, 2011) and hydrolysis of the polyester surface using enzymes and surfactants (Gao et al., 2017). Wu et al. (2014) modified the polyester surface using cutinase treatment. Natarajan and Moses (2012) used polyvinyl alcohol to modify the polyester hydrophilicity. Recently Chen, Haase, and Mahltig (2019) modified a polyester fabric surface using a silica-chitosan composite, which succeeded in enhancing the hydrophilicity of the polyester fabric surface by measuring the sink-in time. They use tetraethoxysilane (TEOS) as primary precursors for preparing a spherical shape of silica nanoparticles. However, considering the material cost, alkoxide may not be commercially viable in the textile industry.
Sodium silicate has been
considered as an alternative inexpensive silica precursor to substitute
expensive alkoxide compounds (Hwang,
Lee, and Chun, 2021). Researchers have
prepared diverse nanostructured silica from sodium silicate (Owoeye,
Abegunde,
and Oji, 2021; Chiang et al., 2017, 2011; Jesionowski and
Krysztafkiewicz, 2002). However, they synthesized sodium silicate
precursor in an alkaline medium to produce non-agglomerated silica
nanoparticles. In contrast, the synthesis of silica from sodium silicate in
acid conditions tends to result in agglomeration (Zulfiqar,
Subhani, and Husain, 2016).
Chitosan is a unique
cationic polysaccharide that can easily be modified with chemicals, radiation,
and enzymes (Barleany et al.,
2020; Usman et al., 2018). It is a
biodegradable biopolymer used in an environmentally friendly synthesis process (Lim et al.,
2021; Usman et al., 2018;
Kusrini et al., 2015). As a
biopolymer, chitosan is a useful polymer for synthesizing metal oxide and
encapsulating small particles (Lim et al.,
2021; Matusiak, Grzadka, and
Bastrzyk, 2018). Recent
interest in chitosan has increased due to its structure's presence of main
amino groups that acts as crosslinker (Chen, Haase, and Mahltig, 2019; Haerudin et al.,
2010).
This study is aimed to synthesize
silica-based chitosan nanocomposites using the sol-gel method from sodium
silicate and chitosan. The relationship between pH and chitosan concentration
in forming silica-chitosan composites was investigated. The effects of
silica-chitosan nanocomposite coating on the polyester fabric surface were
demonstrated.
2.1.
Material
Knitted polyester fabric having a single
jersey knitted design, mass per unit area 150.6 g/m2, thickness 0.57
mm, yarn count 9.61 tex, 51 courses per inch, 43.67 wales per inch was used as
a substrate for coating. The fabric samples were knitted on a single Fukuhara
machine from 100% polyester filament yarn. Ethanol (99.9%) and acetic acid
(98%) were obtained from Merck. Sodium silicate, chitosan, and distilled water
were obtained from a local market. All chemical reagents were used without
further purification.
2.2.
Preparation of precipitated silica-chitosan
Precipitated silica-chitosan was
prepared by mixing sodium silicate with ethanol and distilled water under
continuous magnetic stirring. The solution was then divided into three equal
parts. Acetic acid was titrated to each solution to adjust the pH value to 3,
4, and 5. Chitosan solution with concentrations of 0%, 0.5%, 1%, and 1.5% was
added to the different pH of sodium silicate solutions. Finally, white
precipitated silica-chitosan was collected by centrifugation and filtering and
then dried in an electric oven at 50oC for 2 h.
2.3.
Coating silica-chitosan on polyester fabric
Five pieces of polyester fabrics were
coated with silica-chitosan nanocomposite using a pad-dry-cure method. The
polyester fabric was padded for double-nip and double-dip in a 100 mL bath with
a wet pick-up ratio of 70%. The silica-chitosan-treated polyester fabric was
then dried at 100oC for 1 min and cured at 150oC for 2
min.
2.4. Characterization of silica-chitosan
nanocomposite
The silica-chitosan nanocomposite and
coated fabric surface morphology were observed using a Hitachi TM SU-3500
Scanning electron microscope (SEM). The functional groups of nanocomposites
were analyzed using a Shimadzu Prestige 21 Fourier-transform infrared (FTIR)
Spectrophotometer. The microstructure of the samples was measured using a
Bruker X-ray diffractometer (XRD) at 40 kV with a radiation source
2.5.
Characterization of the coated fabric surface
The polyester fabric surface was
evaluated, including water contact angle and absorption. The wettability or
absorbency of polyester fabrics was examined before and after treatment using
the AATCC test method 79 (AATCC, 2018). In brief, a drop of water is dropped from a fixed height onto the
surface of a test specimen. The time required for the specular reflection of
the water drop to disappear is measured and recorded as sink-in time. The water
contact angle on polyester fabric before and after treatment was measured using
a Kyowa KYOWA interFAce Measurement and Analysis System. The volume of the
water droplet was set at
3.1. The morphology of silica-chitosan
nanocomposites
SEM examined the surface
morphology of the silica and silica-chitosan products; representative images
are shown in Figure 1. At pH 3, the silica product synthesized without chitosan
(pH 3 Ch 0) depicted a loose spherical particle of various sizes from 50 nm to
200 nm. With increasing chitosan concentration (pH 3 Ch 0.5 – 1.5), the surface
morphology did not change, but the silica-chitosan product decreased the size
(96.0 nm – 120.3 nm) and was narrowly distributed. The surface morphology of
the silica-chitosan product prepared at pH 4 and 5 have the same trend as the
surface morphology of the silica-chitosan product prepared at pH 3.
Figure 1 SEM images of silica and silica-chitosan particles
synthesized at different pH and chitosan concentrations
To reveal the particle size distribution
of the silica and silica-chitosan, the particle images in Figure 1 were
analyzed using ImageJ software. Figure 2 shows the particle size distribution
of the silica and silica-chitosan products prepared at pH 3 – 5. It can be seen
from Figure 2 that the silica and silica product prepared at low pH tends to
have a narrow particle size distribution. This is because the pH value strongly
influences the hydrolysis and condensation reactions in the sol-gel process.
Under acidic conditions, the hydrolysis reaction rate is faster than the
condensation reaction rate to produce a small particle size (Barker et.al.,
2022; Budnyak et al., 2015). Therefore, the average particle size of the silica
(119.5 nm) and silica-chitosan (96 - 120.3 nm) compared at pH 3 is smaller than
the silica (182 nm and 165.5 nm) and silica-chitosan (126.6 – 144.2 nm and
177.6 – 201.4 nm) prepared at pH 4 and 5, respectively. It is also clearly
noticed in Figure 2 that the average size of silica prepared without chitosan
is larger than that prepared by adding chitosan. Increasing the concentration
of chitosan tends to enhance the size of silica-chitosan. The higher the concentration
of chitosan involved in the reaction, the larger the size of the
silica-chitosan formed. In an acidic environment, the chitosan acts as a
crosslinker that connects the formed silica particles (Pierog, Gierszewska-Druzynska, and Ostrowska-Czubenko, 2009). The amine group of chitosan (NH2) is
protonated to be positively charged to form -NH3+ ion,
while silica is negatively charged. The interactions between the negatively
charged silicon oxide surface and the positively charged polymer chains formed
silica-chitosan nanocomposite (Chen,
Haase,
and Mahltig, 2019; Budnyak et al., 2015).
Figure 2 Particle size distribution of silica and
silica-chitosan prepared at (a) pH 3, (b) pH 4, and (c) pH 5
3.2. FTIR of silica-chitosan nanocomposites
Figure 3 shows the FTIR spectra of the
silica and silica-chitosan nanocomposite with chitosan concentration and pH
variations. The FTIR spectra pose similar peak vibrations for all samples,
indicating that changing pH and adding chitosan does not alter the silica
backbone. A large vibration area peak at 3424 cm-1 corresponds to
O-H and N-H stretching. A small peak at 1638 cm-1 relates to the
possible interaction between the hydroxyl group of silica and the amine group
of chitosan (Imani et
al., 2022). Sharp
peaks at 1092 cm-1 and 789 cm-1 are attributed to
asymmetric and symmetric stretching vibration Si-O-Si. The peak at 941 cm-1
indicates a functional group of Si-OH stretching vibration (Ryu
and Lee, 2018; Shariatinia and Jalali, 2018), and the
peak at 457 cm-1 indicates the existence of O-Si-O symmetric
deformation vibration (Mahmoud
et al., 2012).
Figure 3 FTIR spectra of
silica and silica-chitosan
The
silica and silica-chitosan product synthesized at pH 3 have relatively the same
peak intensities, while the samples synthesized at pH 4 and 5 have changed the
peak intensities. Therefore, peak intensities of OH vibration change slightly
in the sample. Chitosan is easily soluble in acidic solutions with a pH of less
than 6. At pH 2-3, silica has a zero charge point where the concentration of
the positively charged group is the same as the negative charge. Therefore, it
is possible that at pH 3, even though different concentrations of chitosan are
added, the intensity in the FTIR spectra is not changed. In acid conditions,
the silica surface is generally negatively charged (Matusiak,
Grzadka, and Bastrzyk, 2018). At pH 4
and 5, silica surfaces tend to be more negatively charged than at pH 3.
On the other hand, the polymer chain of
chitosan has a positively charged -NH3+ group. The
interaction between the negatively charged SiO2 surface and the
positively charged chitosan became possible due to the increasing negative
charge on the silica surface at pH 4 and 5 and the increasing positive charge
from adding chitosan. As a result, the intensity of the OH and Si-O-Si, Si-OH,
and O-Si-O groups in the FTIR spectrum changes.
3.3.
Morphology of the silica-chitosan coated polyester fabric
The polyester fabrics were coated with
silica-chitosan nanocomposite using a pad-dry-cure method. The surface
morphology of the untreated and treated polyester fabric is shown in Figure 4.
The optimal condition was determined to
be pH 3 with 0.5 % chitosan (pH 3 Ch 0.5) based on an analysis of the
morphology of the silica chitosan nanocomposite, particle distribution, and
functional groups. It shows the presence of a layer on the surface of the
fabric coated with silica-chitosan. The presence of a layer on the surface of
the polyester affects the surface roughness, so it influences its wetting
ability.
The presence of silica-chitosan particle
aggregates on the polyester fabric surface suggests that silica-chitosan
nanocomposites adhere to the polyester fabric surface, most likely by van der
Waals interactions. The polyester chain has a hydroxyl group at the end,
enabling it to bond to the hydroxyl group of the silica-chitosan.
Figure 4 SEM images of (a) untreated polyester fabric surface and treated
polyester fabric surface at pH 3 with (b) no chitosan, with chitosan of (c)
0.5%, (d) 1.0%, and (e) 1.5%
Figure
5 shows a prediction of the reaction between silica and silica chitosan
nanocomposite in the polyester layer. As
predicted in the reaction, the hydroxyl group on silica (Si-OH) will bond to
the polyester hydroxyl group at the end of the polymer chain (a). Figure b
illustrates the hydroxyl group of chitosan bonds to the hydroxyl group of
silica (Si-OH), and the hydroxyl group of silica bonds to the hydroxyl group of
polyester after forming the silica chitosan nanocomposite. These findings
indicate that the addition of hydroxyl groups to the surface of polyester can
enhance its hydrophilic characteristics.
Figure 5 Schematic representation of the proposed reaction of polyester fabric
surface with (a) silica nanocomposite, (b) silica-chitosan nanocomposite
3.4.
Measurement of static and dynamic contact angles
Figure 6 shows images of water droplets
on polyester fabric surfaces before and after treatment with silica-chitosan
sol. The static contact angle was measured 3 seconds after a water droplet of was placed on the fabric surface. Figure 6(a) shows the static contact angle
of water droplets on untreated fabric is 122o. While dropping water
droplets on treated fabric with silica-chitosan 0 – 1.5% resulted in zero
degrees contact angle, which suggests that coating fabric with silica or
silica-chitosan produced a hydrophilic polyester surface. Furthermore, the
hydrophilicity of treated polyester with silica and silica-chitosan was tested
by measuring the time elapse for a water droplet to disappear or be completely
absorbed by the fabric.
Figure 6 Water droplets on the polyester fabric surface, (a) static, and (b)
dynamic contact angle
Figure
6(b) shows time elapses for a water droplet to disappear from the untreated and
treated fabric surface. For the untreated fabric, the water droplet formed a
ball-like water droplet with a contact angle of 1220. The ball-like
water droplet remained unchanged for 60 seconds, and then the water droplet
suddenly disappeared. On the other hand, the treated fabric absorbed the water
droplet as the droplet immediately touched the fabric surface. It requires 9
seconds for the water droplet to absorb completely into the fabric, while the
silica-chitosan-treated fabric requires 2-4 seconds. It suggests that coating
fabric with silica-chitosan enhances surface energy and hydrophilicity.
Chitosan and sodium silicate were used to create a silica-chitosan
nanocomposite. This research investigated the morphology, particle
distribution, functional groups, and prospective applications of
silica-chitosan nanocomposites in modified polyesters. The pH condition and the chitosan concentration affected particle growth.
The optimal conditions for making nanocomposites during the synthesis process
were pH 3 and chitosan 0.5%. The application of a silica-chitosan
nanocomposite coating to polyester fabrics has been proven to enhance the
hydrophilic properties of polyester. Surface modification with silica-chitosan
nanocomposites provides an opportunity in the process of functional textile
modification but also presents a challenge for future research on the
application of bio nanocomposites in the textile industry.
The author would
like to thank Mr. Rifki Septawendar and Mrs. Wiah Wardiningsih for their
contributions that have helped us during the research and discussion. This
research was funded by the Ministry of Industry of the Republic of Indonesia as
part of a program for doctoral fellowships (Beasiswa S3
Mandiri BPSDMI,2018).
AATCC, 2018. AATCC Test Methode 79, Test Method for Absorbency of Textiles
Barker, C.R., Lewns, F.K.,
Poologasundarampillai, G. and Ward, A.D., 2022, In Situ Sol-Gel Synthesis of Unique Silica Structures Using Airborne
Assembly: Implications for In-Air Reactive Manufacturing, ACS Applied Nano
Materials, Volume 5(8), pp. 11699–11706
Barleany, D.R., Ananta, C.V.,
Maulina, F., Rochmat, A., Alwan, H., Erizal, E., 2020. Controlled Release of
Metformin Hydrogen Chloride from Stimuli-Responsive Hydrogel Based on Poly(N-Isopropylacrylamide)/Chitosan/Polyvinyl
Alcohol Composite. International Journal of Technology, Volume 11(3), pp. 511–521
Budnyak, T.M., Pylypchuk, I. V., Tertykh, V.A., Yanovska, E.S., Kolodynska,
D., 2015. Synthesis and Adsorption
Properties of Chitosan-Silica Nanocomposite Prepared by Sol-Gel Method. Nanoscale
Research Letters, Volume 10(1), pp. 1–10
Chen, G., Haase, H., Mahltig, B., 2019. Chitosan-Modified Silica Sol
Applications for The Treatment of Textile Fabrics: A View on Hydrophilic,
Antistatic and Antimicrobial Properties. Journal of Sol-Gel Science and
Technology, Volume 91(3), pp. 461–470
Chiang, W.Y., Usman, A., Sugiyama, T., Hofkens, J., Masuhara, H., 2017. Femtosecond Laser Trapping Dynamics of
Nanoparticles: A Single Transient Assembly Formation Leading to Their
Directional Ejection. Journal of Physical Chemistry C, Volume 122(25),
pp 13233–13242
Chiang, Y.D., Lian, H.Y., Leo, S.Y., Wang, S.G., Yamauchi, Y., Wu,
K.C.W., 2011. Controlling Particle Size and Structural Properties of
Mesoporous Silica Nanoparticles Using The Taguchi Method. Journal of
Physical Chemistry C, Volume 115(27), pp. 13158–13165
Gao, A., Shen, H., Zhang, H., Feng, G., Xie, K., 2017. Hydrophilic Modification of Polyester
Fabric by Synergetic Effect of Biological Enzymolysis and Non-Ionic Surfactant,
and Applications in Cleaner Production. Journal of Cleaner Production,
Volume 164, pp. 277–287
Haerudin, H., Pramono, A.W., Kusuma, D.S., Jenie, A., Voelcker,
N.H., Gibson, C., 2010. Preparation and Characterization of Chitosan/Montmorillonite
(MMT) Nanocomposite Systems. International Journal of Technology, Volume
1(1), pp. 65–73
Hwang, J., Lee, J.H., Chun, J., 2021. Facile Approach for The Synthesis of
Spherical Mesoporous Silica Nanoparticles from Sodium Silicate. Materials
Letters, Volume 283, p. 128765
Imani, N.A.C., Kusumastuti, Y., Petrus, H.T.B.M., Timotius, D.,
Putri, N.R.E., Kobayashi, M., 2022. Preparation, Characterization, and
Release Study of Nanosilica/Chitosan Composite Films. International Journal
of Technology, Volume 13(2), pp. 444–453
Jesionowski, T., Krysztafkiewicz, A., 2002. Preparation of The
Hydrophilic/Hydrophobic Silica Particles. Colloids and Surfaces A:
Physicochemical and Engineering Aspects, Volume 207(1-3), pp. 49–58
Kusrini, E., Shiong, N.S., Harahap, Y., Yulizar, Y., Arbianti, R., Pudjiastuti,
A.R., 2015. Effects of Monocarboxylic Acids and Potassium Persulfate on
Preparation of Chitosan Nanoparticles. International Journal of Technology,
Volume 6(1), pp. 11–21
Lim, M.J., Shahri, N.N.M., Taha, H., Mahadi, A.H., Kusrini, E.,
Lim, J.W., Usman, A., 2021. Biocompatible Chitin-Encapsulated Cds Quantum Dots: Fabrication
and Antibacterial Screening. Carbohydrate Polymers, Volume 260, p. 117806
Mahmoud, M.M., Folz, D.C., Suchicital, C.T., Clark, D.E., 2012. Crystallization of Lithium Disilicate
Glass Using Microwave Processing. Journal of the American Ceramic Society,
Volume 95(2), pp. 579–585
Matusiak, J., Grzadka, E., Bastrzyk, A., 2018. Stability, Adsorption and Electrokinetic
Properties of The Chitosan/Silica System. Colloids and Surfaces A: Physicochemical
and Engineering Aspects, Volume 554, pp. 245–252
Natarajan, S., Moses, J.J., 2012. Surface Modification of Polyester Fabric
Using Polyvinyl Alcohol in Alkaline Medium. Indian Journal of Fibre and
Textile Research, Volume 37(3), pp. 287–291
Owoeye, S.S., Abegunde, S.M., Oji, B., 2021. Effects of Process Variable on Synthesis and
Characterization of Amorphous Silica Nanoparticles Using Sodium Silicate
Solutions as Precursor by Sol–Gel Method. Nano-Structures & Nano-Objects,
Volume 25, p. 100625
Pierog, M., Gierszewska-Druzynska, M., Ostrowska-Czubenko, J., 2009. Effect of Ionic Crosslinking Agents on Swelling
Behaviour of Chitosan Hydrogel Membranes. Progress on Chemistry and
Application of Chitin and Its
Derivatives, Volume 75(82), p. 3
Ramakrishnan, V., Jagannathan, S., 2018. Design of Polyester-Elastane-Cotton
Plated Knitted Components for Hot and Dry Environment. International Journal
of Clothing Science and Technology, Volume 30(5), pp. 657–667
Ryu, Y.B., Lee, M.S., 2018. Infrared spectra and Thermal Properties of
Sodium Silicate Solutions: Molecular Binding Structure and Thermal Properties of
Sodium Silicate Aqueous Solution. Journal of Korean Institute of Metals and
Materials, Volume 56(1), pp. 72–78
Shariatinia, Z., Jalali, A.M., 2018. Chitosan-Based Hydrogels: Preparation,
Properties and Applications. International Journal of Biological
Macromolecules, Volume 115, pp. 194–220
Shishoo, R., 2005. Introduction. In: Textiles in Sport,
Shishoo, R., (ed.), CRC Press, Boca Raton, Boston, New York, Washington, DC,
USA, pp. 1-8
Troynikov, O., Wardiningsih, W., 2011. Moisture Management Properties of Wool/
Polyester and Wool/Bamboo Knitted Fabrics for The Sportswear Base Layer. Textile
Research Journal, Volume 81(6), pp. 621–631
Usman, A., Kusrini, E., Widiantoro, A.B., Hardiya, E., Abdullah,
N.A., Yulizar, Y., 2018. Fabrication of Chitosan Nanoparticles Containing Samarium Ion
Potentially Applicable for Fluorescence Detection and Energy Transfer. Chemical
Engineering, Volume 9(6), pp. 1112–1120
Wu, J., Cai, G., Liu, J., Ge, H., Wang, J., 2014. Eco-Friendly Surface Modification on
Polyester Fabrics by Esterase Treatment. Applied Surface Science, Volume
295, pp. 150–157
Zaman, M., Liu, H., Xiao, H., Chibante, F., Ni, Y., 2013. Hydrophilic Modification of Polyester Fabric by Applying Nanocrystalline Cellulose Containing Surface Finish. Carbohydrate Polymers, Volume 91(2), pp. 560–567
Zulfiqar, U., Subhani, T., Wilayat Husain, S., 2016. Synthesis of Silica Nanoparticles from Sodium Silicate under Alkaline Conditions. Journal of Sol-Gel Science and Technology, Volume 77(3), pp. 753–758
