Investigation on Saprolitic Laterite Ore Reduction Process using Palm Kernel Shell Charcoal: Kinetics and Phase Transformation
Corresponding email: bayupetrus@ugm.ac.id
Published at : 01 Jul 2022
Volume : IJtech
Vol 13, No 3 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i3.4701
Petrus, H.T.B.M., Putera, A.D.P., Warmada, I.W., Nurjaman, F., Astuti, W., Prasetya, A., 2022. Investigation on Saprolitic Laterite Ore Reduction Process using Palm Kernel Shell Charcoal: Kinetics and Phase Transformation. International Journal of Technology. Volume 13(3), pp. 565-574
| Himawan Tri Bayu Murti Petrus | 1. Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Yogyakarta 55281, Indonesia 2. Unconventiona |
| Andreas Diga Pratama Putera | Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Yogyakarta 55281, Indonesia |
| I Wayan Warmada | 1. Unconventional Geo-resources Research Center, Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Yogyakarta 55281, Indonesia 2. Department of Geological Engineering, Faculty of En |
| Fajar Nurjaman | Research Unit for Mineral Technology, Indonesian Institute of Sciences (LIPI), Jl. Ir. Sutami Km. 15, Tanjung Bintang, Lampung Selatan, Indonesia |
| Widi Astuti | Research Unit for Mineral Technology, Indonesian Institute of Sciences (LIPI), Jl. Ir. Sutami Km. 15, Tanjung Bintang, Lampung Selatan, Indonesia |
| Agus Prasetya | 1. Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Yogyakarta 55281, Indonesia 2. Unconventiona |
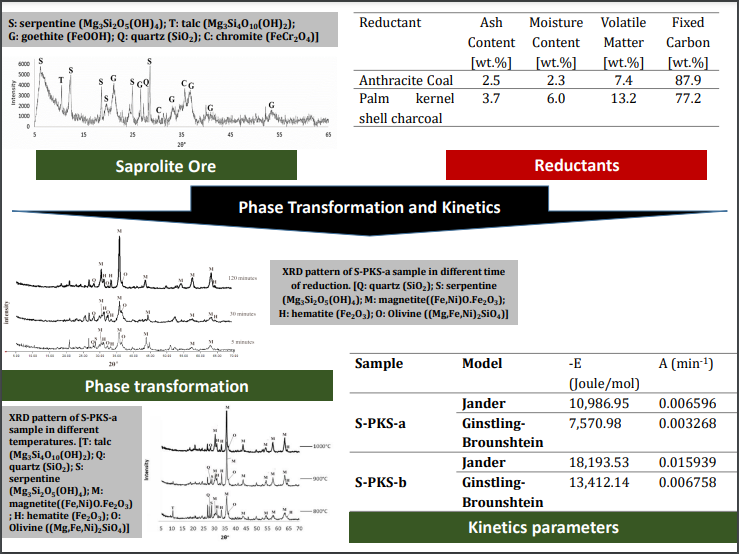
The performance and kinetic of saprolitic laterite reduction using palm
kernel shell charcoal and anthracite were studied. The anthracite coal
represents the conventional high-grade carbon content matter, and palm kernel
shell charcoal represents biomass-based reductant. The experiment was conducted
at a temperature ranging from 800oC and 1000oC. XRD analysis was applied to
observe phase transformation. For the kinetic study, two models, namely (1)
Jander and (2) Ginstling-Brounhstein diffusion model, were applied. The mineral
phase results indicated that both reductants yield Magnetite from Goethite in
the laterite. The best fit model is obtained by the Jander model with the
energy activation of 33.68 kJ/mol for anthracite reductant and
10.99 – 18.19 kJ/mol for palm kernel shell reductant, indicating that reduction
is easier to occur using palm kernel shell.
Kinetics; Phase transformation; Reduction; Roasting; Saprolite
Nickel could be a transition component
with properties of ferrous and nonferrous metals (Kim
et al., 2010). Nickel ore is affiliated with oxide (nickel laterite) or
sulfur (nickel sulfide). Almost 58% of nickel requests are provided by sulfide
metals, even though 78% of nickel is stored in laterite minerals (Dalvi et al., 2004). However, as the continuous
exploitation of sulphidic ores occurred in recent years, the sources became
scarce and underground mining was introduced. Consequently, the exploitation
cost was rising, especially the labour cost. On the contrary, the mining activity
of laterite deposits is considerably shallow (usually less than 50 meters) (Elias, 2002). So, much concern has been
concentrated on using low-grade nickel ore (especially those containing <2.0
wt.% nickel) (Lee et al., 2005), such as
laterites.
In terms of nickel laterite, Indonesia has an abundant deposit of
it. About 12% of nickel
There are two kinds of laterite, namely limonite and saprolite. Limonite is
low-nickel content laterite (around 0.8-1.5% Ni-mass), and saprolite is a rich-nickel content
(more than 1.5-3%
Ni-mass) (Whittington
& Muir, 2000). Both
hydrometallurgical and pyrometallurgical processes can be used to extract
nickel from the laterites. However, due to its high nickel content, saprolite
ore is better
processed by pyrometallurgy
(Li et al., 2011; Minister of Energy and Mineral Resources Republic
of Indonesia, 2013). There are usually
three unit operations in the pyrometallurgical process: roasting, smelting, and
converting. The reduction process consumes carbon-based reductant, usually coke,
and produces a tremendous amount of carbon dioxide. This process is highly
energy-consuming (Guo et al.,
2009) and not
environmentally friendly.
Replacing the coke with
bio-reductant has been an interesting issue concerning carbon dioxide emission
to be studied.
Palm kernel shell charcoal is the
potential to be utilized as a reductant in the saprolite roasting process. The
saprolite sample mixed with palm kernel shell charcoal can yield the identical
mineral phase to the sample that used anthracite coal after the roasting
process, namely Magnetite, Olivine, and Hematite. The product minerals are
transformed from Goethite, confirming the reduction in all samples. In
addition, higher temperature and prolonged reduction process increase the
conversion of Goethite into magnetite. In terms of kinetics results, the Jander
model fits better for both reductants than the Ginstling-Brounshtein model,
with lower energy activation for the biomass-based reductant of 10.99 – 18.19
kJ/mol compared to that of anthracite reductant of about 33.68 kJ/mol. The
positive results of biomass-based charcoal utilization hopefully encourage the
development of a sustainable pyro-based nickel laterite processing process.
We highly appreciate the Ministry of
Research, Technology and Higher Education of Indonesia for the financial
support in the scheme of PUPT (Penelitian Unggulan Perguruan Tinggi) 2364/UN1.P.III/DIT-LIT/LT/2017
and BPDP Kelapa Sawit Research Grant for Students. On behalf of all authors,
the corresponding author states that there is no conflict of interest.
Chen, G..,
Hwang, W., Liu, S., Shiau, J.,
2015. The Effect oF Bio-Coal on the on the Carbothermic Reduction of Laterite
'Ores', Materials Transactions, Volume 56(4), pp. 550–555
Citrawati, F., Dwiwandono, R., Firmansyah, L. 2020. The Effect
of Ni on the Formation of Bainite in Fe-Ni Lateritic Steels Through Semi-Continuous
Cooling Method. International Journal of Technology, Volume 11(1), pp.
60–70
Dalvi, A.D., Bacon, W.G., Osborne, R.C., 2004. The Past and
the Future of Nickel Laterites World's Land Based Nickel Resources and Primary
Nickel Production Nickel Production. kt / 'yr', PDAC 2004 International
Convention, pp. 1–27
Elias, M., 2002. Nickel Laterite Deposits–Geological
Overview, Resources and Exploitation. Centre
for Ore Deposit Research, University of Tasmania, Special Publication, pp.
205-220
Fu, J.X., Zhang, C.,
Hwang, W., Liu, Y., Lin, Y., 2012. Exploration of Biomass Char for CO2
Reduction in RHF Process For Steel Production. International Journal of
Greenhouse Gas Control, Volume 8, pp. 143–149
Guo, X., Li, D., Park, K., Tian, Q., Wu, Z., 2009. Leaching Behavior
of Metals from a Limonitic Nickel Laterite using a Sulfation-Roasting-Leaching
Process. Hydrometallurgy, Volume 99(3–4), pp. 144–150
Kartohardjono, S., Pamitran, A.S., Putra, N., 2019. Biomass:
from Waste to Valuable Materials. International Journal of Technology,
Volume 10(8), pp. 1465–1468
Khawam, A., Flanagan, D.R., 2006. Solid-State Kinetic Models:
Basics and Mathematical Fundamentals. Journal of Physical Chemistry B, Volume
110(35), pp. 17315–17328
Kim, J., Dodbiba, G., Tanno, H., Okaya, K., Matsuo, S.,
Fujita, T., 2010. Calcination of Low-Grade Laterite for Concentration of Ni by
Magnetic Separation. Minerals Engineering, Volume 23(4), pp. 282–288
Lee, H.Y., Kim, S.G., Oh, J.K., 2005. Electrochemical Leaching
of Nickel from Low-Grade Laterites. Hydrometallurgy, Volume 77(3–4), pp.
263–268
Li, B., Wang, H., Wei, Y., 2011. The Reduction of Nickel from
Low-Grade Nickel Laterite Ore using a Solid-State Deoxidisation Method. Minerals
Engineering, Volume 24(14), pp. 1556–1562
Li, Y.J., Sun, Y., Han,
Y., Gao, Y., 2013. Coal-Based Reduction Mechanism of Low-Grade Laterite Ore. Transactions
of Nonferrous Metals Society of China, Volume 23(11), pp. 3428–3433
Minister of
Energy and Mineral Resources Republic Of Indonesia, 2013. Regulation Of The Minister Of Energy And
Mineral Resources Republic Of Indonesia No 20. Indonesia. Available online
at http://Jdih.Esdm.Go.Id/Peraturan/Permen ESDM 20 2013. pdf.
Mondal, K., Lorethova, H., Hippo, E., Wiltowski, T., Lalvani,
S.B., 2004. Reduction of Iron Oxide in Carbon Monoxide Atmosphere-Reaction Controlled
Kinetics. Fuel Processing Technology, Volume 86(1), pp. 33–47
Oediyani, S., Willyandhika, K., Suharto. 2016. The Effect of
Reduction Time and Size Distribution of Mixed Iron Ore with Coconut Shell
Charcoal on the Percentage Of Metallization by using a Rotary Kiln. International
Journal of Technology, Volume 7(3), pp. 366–373
Petrus, H.T.B.M., Rhamdani,
A.R., Putera, A.D.P., Warmada, I.W., Yuliansyad, A.T., Perdana, I., 2016.
Kinetics Study of Carbon Raiser on the Reduction of Nickel Laterite from Pomalaa,
Southeast Sulawesi. In: IOP Conference Series: Materials Science and
Engineering, Volume 162, Second International Conference on Chemical
Engineering (ICCE) UNPAR 26–27 October 2016, Bandung, Indonesia
Petrus, H.T.B.M., Putera,
A.D.P., Rhamadani, A., Warmada, I.W., Yuliansyad, A.T., Perdana, I., 2017.
Lamtoro Charcoal (L. Leucocephala) as Bioreductor in Nickel Laterite Reduction:
Performance and Kinetics Study. In: 2nd International Symposium on Frontier of
Applied Physics (ISFAP 2016) 3–5 October 2016, Jakarta, Indonesia
Petrus, H.T.B.M., Putera,
A.D.P., Sugiarto, E., Perdana, I., Warmada, I.W., Nurjaman, F., Astuti,
W., Mursito, A.T., 2019. Kinetics on Roasting Reduction oLimonitic Laterite Ore
using Coconut-Charcoal and Anthracite Reductants. Minerals Engineering, Volume
132(2), pp. 126–133
Putera, A.D.P., Sugiarto, E., Sutijan, Warmada, I.W., Petrus,
H.T.B.M., 2017. Coconut Shell Charcoal as a Bioreductor in Roasting Process of
Nickel Laterite from Pomalaa, Southeast Sulawesi: Performance and Kinetics
Study. Materials Science Forum, Volume 901, pp. 182–189
Rasyid, M.H. Al., Rhamdani, A.R., Putera, A.D.P., Putrus,
H.T.B.M., 2016. Study on Biomass Performance in Reduction of Nickel Laterite
from Pomalaa, Sulawesi Tenggara. In: AIP Conference Proceedings 1755, p. 050007
Rhamdhani, M.A., Hayes, P.C., Jak, E., 2009. Nickel Laterite
Part 1-Microstructure and Phase Characterisations During Reduction Roasting and
Leaching. Transactions of the Institutions of Mining and Metallurgy, Section
C: Mineral Processing and Extractive Metallurgy, 118(3), pp. 129–145
Ribeiro, P.P.M.,
Neumann, R., Santos, I.D.D., Rezende, M.C., Radino-Rouse, P., Dutra, A.J.B.,
2019. Nickel Carriers in Laterite Ores and Their Influence on the Mechanism of
Nickel Extraction by Sulfation-Roasting-Leaching Process. Minerals
Engineering, 131, pp. 90–97
U.S. Geological
Survey, 2014. Mineral Commodity Summaries 2014: U.S. Report, Geological Survey,
USA
Whittington, B.I. Muir, D., 2000. Pressure Acid Leaching of
Nickel Laterites: A Review. Mineral Processing and Extractive Metallurgy
Review, Volume 21(6), pp. 527–599
Yunus, N.A., Ani, M.H., Salleh, H.M., Rashid, R.Z.A.,
Akiyama, T., Purwanto, H., Othman, N.E.F.,
2014. Effect of Reduction Roasting by using Biochar Derived from Empty Fruit
Bunch on the Magnetic Properties of Malaysian Iron Ore. International
Journal of Minerals, Metallurgy and Materials, Volume 21(4), pp. 326–330
