The Formation Process of Hydroxyapatite Nanoparticles by Electrolysis and Their Physical Characteristics
Corresponding email: supriyono16@ub.ac.id
Published at : 04 Apr 2023
Volume : IJtech
Vol 14, No 2 (2023)
DOI : https://doi.org/10.14716/ijtech.v14i2.4452
Supriyono, Kartikowati, C.W., Poerwadi, B., Wulandari, C., Hikma, L.L.F., Azzahra, A., Ghanyysyafira, K., Pinastika, H.L., 2023. The Formation Process of Hydroxyapatite Nanoparticles by Electrolysis and Their Physical Characteristics. International Journal of Technology. Volume 14(2), pp. 330-338
| Supriyono | Chemical Engineering Department, Faculty of Engineering, Universitas Brawijaya, Jl. MT. Haryono No. 167, Malang 65145, Indonesia |
| Christina Wahyu Kartikowati | Chemical Engineering Department, Faculty of Engineering, Universitas Brawijaya, Jl. MT. Haryono No. 167, Malang 65145, Indonesia |
| Bambang Poerwadi | Chemical Engineering Department, Faculty of Engineering, Universitas Brawijaya, Jl. MT. Haryono No. 167, Malang 65145, Indonesia |
| Chindy Wulandari | Chemical Engineering Department, Faculty of Engineering, Universitas Brawijaya, Jl. MT. Haryono No. 167, Malang 65145, Indonesia |
| Lyla Liilia Fitria Hikma | Chemical Engineering Department, Faculty of Engineering, Universitas Brawijaya, Jl. MT. Haryono No. 167, Malang 65145, Indonesia |
| Aulia Azzahra | Chemical Engineering Department, Faculty of Engineering, Universitas Brawijaya, Jl. MT. Haryono No. 167, Malang 65145, Indonesia |
| Kharisma Ghanyysyafira | Chemical Engineering Department, Faculty of Engineering, Universitas Brawijaya, Jl. MT. Haryono No. 167, Malang 65145, Indonesia |
| Hira Listya Pinastika | Chemical Engineering Department, Faculty of Engineering, Universitas Brawijaya, Jl. MT. Haryono No. 167, Malang 65145, Indonesia |
The
electrolysis method for synthesizing hydroxyapatite nanoparticles (NPs) has the
advantage of controlling the particle size by adjusting the potential and
current used. This study aims to study the electrolysis of hydroxyapatite NPs
formation and its characteristics. The solution contained Na2H2EDTA.2H2O,
KH2PO4, and CaCl2, with an EDTA/PO43-/Ca2+
concentration of 0.2/0.2/0.2 M. The electrolytic potential are 4, 5, and 6
volts for 6 hours. The carbon electrode spacing used is 2 cm. The precipitate
formed is filtered with a vacuum jet ejector. Retentate was washed with
demineralized water and dried in an oven at 105oC. The synthesis of pure
hydroxyapatite by electrolysis was successfully carried out at a potential of 5
volts. The OH- ion, which comes from the H2O reduction
process at the cathode, is essential in the formation of brushite, which then
forms hydroxyapatite. The hydroxyapatite, synthesized at a potential of 4
volts, had the smallest particle size (442.4 nm) with the largest particle
surface area (417.22 m2/gram).
Bioceramics; Electrolysis; Hydroxyapatite; Nanoparticles
Hydroxyapatite (HA) particles are a biomaterial having a chemical
formula of Ca10(PO4)6(OH)2. HA has
good biocompatibility and bioactivity properties. The structure of HA
approximates the structure possessed by bones and teeth (Pietrzykowska et al., 2021; Tomozawa and Hiromoto, 2011; Suchanek
and Yoshimura, 1998). HA can bind directly to tissue and stimulate tissue growth. Therefore,
HA has the potential to be applied in the biomedical field, especially for bone
and dental applications (Zhou and Lee, 2011).
HA belongs to the bioceramic
type. In the medical world, ceramic materials are divided into two groups,
namely bioinert ceramics and bioactive ceramics. Bioinert ceramics have no
effect and interact with body tissues (Panda, Biswas, and Paul,
2021). Meanwhile, bioactive ceramics can
bind to living bone tissue, such as HA and calcium phosphate. HA can be used in
a variety of biomedical applications, including matrices for drug release
control (Kamitakahara, Imai, and Ioku, 2013), scaffolds for new bone formation (Rezwan et al., 2006), and
fillers and coatings for repairing osseous damage (Zhou
and Lee, 2011; Banerjee,
Various methods have been
developed to synthesize HA NPs, including precipitation, hydrothermal,
mechano-chemical, flame spray, and electrolysis (Co?rdova-Udaeta
et al., 2021; Lin et al., 2017; Martins et al., 2008; Fathi
and Hanifi, 2007; Chang and Tanaka, 2002). Djosic et al. (2009) have succeeded in synthesizing monetite nanoparticles electrochemically
and transforming them into hydroxyapatite by immersion in NaOH solution.
However, no research has been conducted on the conditions affecting the
electrochemical process to produce hydroxyapatite directly (one step) (Djosic et al., 2009).
Electrolysis is a method that offers an easy and straightforward process (Corona-Gomez, Chen, and Yang, 2016). The particle diameter can be controlled by adjusting
the voltage or current during electrolysis (Djosic
et al., 2009). These results were
obtained from tests with relatively high solution pH and current density.
Theoretically, the higher the pH and current density will accelerate the
particle formation reaction and encourage particle agglomeration (Kim, Kim, and Hirasawa, 2002). If the
pH and current density are relatively high, the pH and current density no
longer affect the particle size but are more influenced by the number of
reactants available and the synthesis time. At relatively low current
densities, these two parameters may have an effect (Montero
et al., 2006). Therefore, this
research focuses on studying the operating conditions, namely the potential for
synthesizing hydroxyapatite NPs.
2.1. Materials and
Instrumentations
The raw materials used in this study
include Na2H2EDTA.2H2O (Merck), KH2PO4
(Merck), CaCl2.7H2O (Merck), and commercial
hydroxyapatite (Merck). While the instrumentation used includes a DC power
supply (GPD X303S, GW Instek), Particle Size Analyzer (Cilas 1190), Surface
Area Analyzer (Quantachrome NOVA 1200), and Powder X-ray Diffraction
(PANalytical X’Pert3 Powder).
2.2. Synthesis
of Hydroxyapatite NPs Powder
3.1. Synthesis
of Hydroxyapatite NPs
Figure 2 shows the synthesized white
powder's X-ray diffraction pattern (XRD). The synthesized white powder shows a
tendency to form hydroxyapatite NPs which is consistent with the peak position
at an angle of 2with JCPDS 03-0747, hexagonal, a = b = 9.4302, c = 6.88911 Å,
space group P63/m. Referring to the reaction, the formation of hydroxyapatite
NPs occurs reversibly; the synthesis of hydroxyapatite NPs requires an optimal
reaction equilibrium (Nur et al., 2014). If
the electrolysis voltage during the hydroxyapatite NPs formation process is set
to the correct value, then the reaction tends to lead to the formation of
hydroxyapatite NPs.
The white powder synthesized from bulk solution at potential 4 Volt has a mixture composition of brushite and hydroxyapatite NPs. The lack of energy used resulted in converting the brushite to hydroxyapatite NPs. The white powder synthesized from bulk solution at a potential of 5 Volts has a pure hydroxyapatite NPs composition. Meanwhile, the white powder which was synthesized from bulk solution at a potential of 6 volts had a dominant composition of hydroxyapatite NPs with a small amount of brushite. This is because the reaction to form hydroxyapatite from brushite is a back-and-forth reaction (Tripathi & Basu, 2012). If the hydroxyapatite NPs still contain brushite, it will reduce their bioactivity when applied medically (Dorozhkin, 2010). The hydroxyapatite formed also has a tendency with the amorphous phase.
Figure 2 The X-ray
diffraction pattern of the synthesized white powder by electrolysis
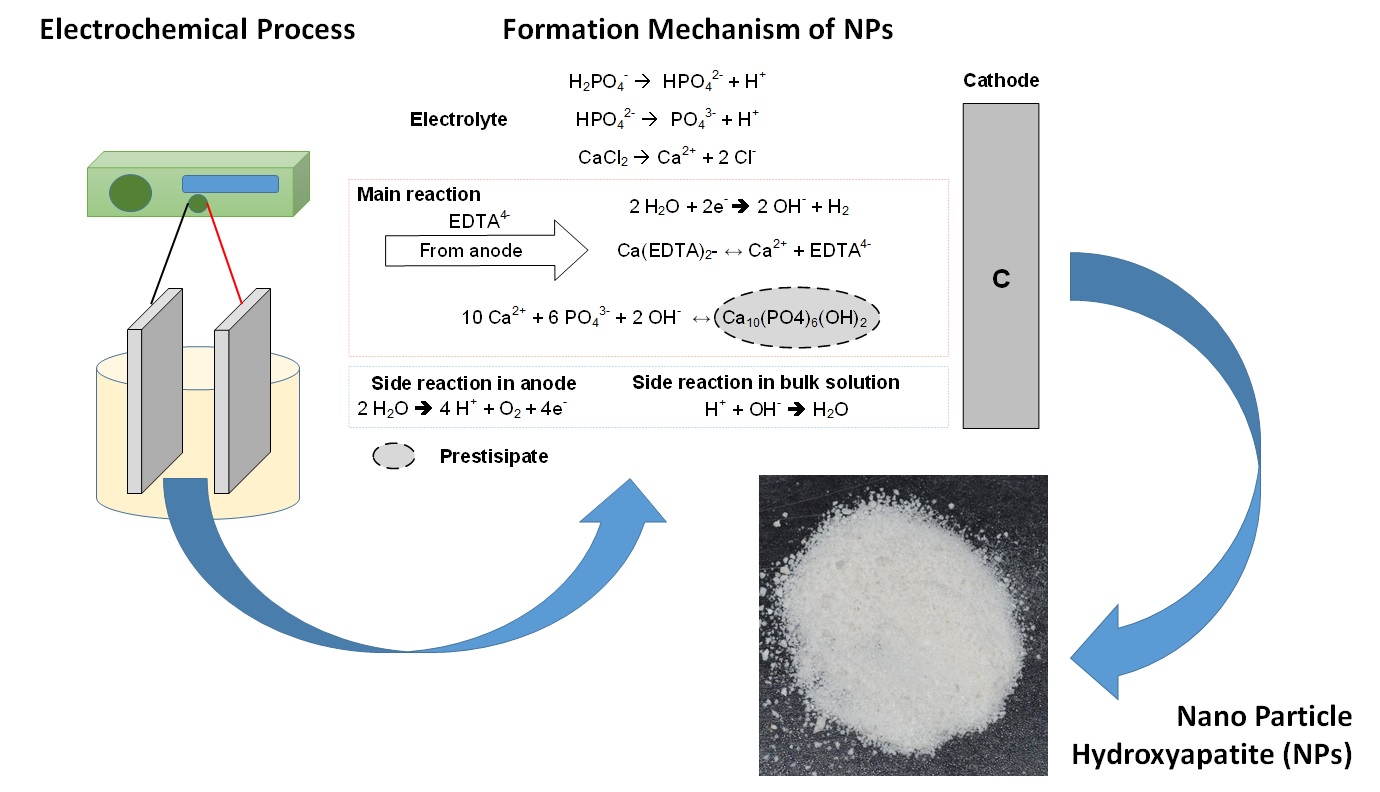
3.2. Reaction
Mechanism for the Formation of Hydroxyapatite NPs
Figure 3
Schematic diagram of the reaction to the electrolytic formation of
hydroxyapatite
Figure 4 Photo of color
change with time of electrolysis of hydroxyapatite NPs by electrolysis
3.3. Distribution
of Hydroxyapatite Nanoparticles Diameter
Figure 5
Size distribution of as-synthesized HA NPs by electrolysis and commercial HA
Figure 6
Schematic diagram of the behavior of hydrogen gas formed on the particle size
of hydroxyapatite
H2 gas in the bulk solution causes an
outward pushing force through the cracks of the hydroxyapatite NPs, causing the
electrolytic synthesized particles to have a smaller size. The white powder
synthesized by electrolysis at a potential of 6 Volt has a more heterogeneous
particle distribution than the particles synthesized at 4 and 5 Volts.
Meanwhile, the distribution of commercial hydroxyapatite particles tends to
have a large distribution range. This is due to particle agglomeration, which
causes a larger range of particle size distribution.
3.4. The Surface Area of the Synthesized Particles
The surface area of the HA NPs showed that the
hydroxyapatite particles synthesized at the 4 Volt potential had a smaller
surface area than those synthesized at the 5 Volt and 6 Volt potentials, which
had almost the same surface area. The particles synthesized with low potential
have better porosity than those synthesized at high potential. This is because
the pores structure of HA NPs
synthesized at the high potential has a tendency to collapse, so that the pore
area tends to be small (Hong et al., 2013). Whereas commercial
hydroxyapatite particles have a smaller surface area than synthetic
hydroxyapatite at a potential of 4 Volts and greater than synthetic
hydroxyapatite at 5 Volts and 6 Volts.
Table 1 The surface area of the
synthesized particles by electrolysis and commercial hydroxyapatite
|
Electrolysis
Potential (Volt) |
Surface
area (m2/gram) |
|
4 |
417.22 |
|
5 |
304.247 |
|
6 |
303.419 |
|
HA
commercial |
355.779 |
Synthesis of pure hydroxyapatite
(100% hydroxyapatite) by electrolysis was successfully carried out at a
potential of 5 volts. The OH- ion, which comes from the H2O
reduction process at the cathode, plays a critical role in the formation of brushite
forming hydroxyapatite. The hydroxyapatite, synthesized at a potential of 4
volts, had the smallest particle size (442.4 nm) with the largest particle
surface area (417.22 m2/gram). Further research needs to examine the
effect of the synthesis time of HA NPs, which is longer than 6 hours with a
voltage of 4 Volts to determine the most effective time variable in the
synthesis of hydroxyapatite.
This work was supported by HPP (HIBAH PENELITI
PEMULA) 2020 through grant Number 436.74/UN10.C10/PN/2020.
Banerjee, A., Bandyopadhyay, A., Bose, S., 2007. Hydroxyapaptite nanopowders: synthesis,
densification and cell materials interaction. Materials Science and
Engineering: C, Volume 27(4), pp. 729–735
Chang, M.C., Tanaka, J., 2002. FT-IR study for
hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials,
Volume 23(24), pp. 4811–4818
Co?rdova-Udaeta, M.,
Kim, Y., Yasukawa, K., Kato, Y., Fujita, T., Dodbiba, G., 2021. Study on the synthesis of hydroxyapatite
under highly alkaline conditions. Industrial & Engineering
Chemistry Research, Volume 60(11), pp. 4385–4396
Corona-Gomez, J., Chen, X., Yang Q., 2016. Effect of
nanoparticle incorporation and surface coating on mechanical properties of bone
scaffold: a brief review. Journal of Functional Biomaterials, Volume
7(3), p. 18
Djosic, M.S.,
Miskovic-Stankovic, V.B., Kacarevic-Popovic, Z.M., Jokic, B.M., Bibic, N.,
Mitric, M., Milonjic, S.K., 2009. Electrochemical
synthesis of nanosized monetite powder and its electrophoretic deposition on
titanium. Colloids and Surfaces A: Physicochemical and Engineering
Aspects, Volume 341(1), pp. 110–117
Dorozhkin, S.V.,
2010. Bioceramics of calcium
orthophosphates. Biomaterials, Volume 31(7), pp. 1465–1485
Fathi, M.H., Hanifi, A., 2007.
Evaluation and chracterization of nanostructure hydroxyapatie powder
prepared by simple sol–gel method. Materials Letters, Volume 61(18), pp. 3978–3983
Hong, X.T., Wu, X.H.,
Mo, M.Y., Luo, Z. P., HUI, K., Chen, H.Y., Zhang, Q.Y., 2013. Synthesis and electrochemical capacitive
performances of novel hierarchically micro-meso-structured porous carbons
fabricated using Microporous rod-like hydroxyapatites as a template. Acta
Physico-Chimica Sinica, Volume 29(2), pp. 298–304
Kamitakahara, M.R., Imai, Ioku, K., 2013. Preparation and evaluation of spherical ca-deficient hydroxyapatite
granules with controlled surface microstructure as drug carriers. Materials
Science and Engineering: C, Volume 33(4), pp. 2446–2450
Kim, W.S., Kim, W.S., Hirasawa, I., 2002. Changes
in crystalline properties of nanosized
hydroxyapatite powders prepared by low-temperature reactive crystallization.
Journal of chemical engineering of Japan, Volume 35(11), pp. 1203–1210
Lin, D.J., Hung F.Y., Lee H.P., Yeh M.L., 2017. Development of a novel degradation-controlled magnesium-based
regeneration membrane for future guided bone regeneration (GBR) therapy.
Metals, Volume 7(11), p. 481
Martins, M., Santos, C.,
Almeida, M., Costa M., 2008. Hydroxyapatite micro and nanoparticles: nucleation
and growth mechanism in the presence of citrate species. Journal of Colloid and Interface
Science, Volume 318(2), pp. 210–216
Montero, M.L., Saenz,
A., Rodriguez, J., Arenas, J., Castano, V.M., 2006. Electro-chemical synthesis of nanoized hydroxyapatite. Journal of materials science, Volume 41(7), pp. 2141–2144
Nasiri-Tabrizi, B.,
Honarmandi, P., Ebrahimi-Kahrizsangi, R., Honarmandi, P., 2009. Synthesis of nanosize single-crystal
hydroxyapatite via mechanochemical method. Materials Letters,
Volume 63(5), pp. 543–546
Nur, A., Setyawan,
H., Widjaja, A., Lenggoro, I. W., 2014. Electrochemical
processes for the formation of hydroxyapatite powders. Bulletin of Chemical
Reaction Engineering & Catalysis, Volume 9(3), pp. 168–175
Panda, S., Biswas, C.K., Paul, S., 2021. A comprehensive review on the preparation and application of calcium
hydroxyapatite: a special focus on atomic doping methods for bone tissue engineering.
Ceramics International, Volume 47(20), pp. 28122–28144
Pietrzykowska, E.,
Romelczyk-Baishya, B., Chodara, A., Koltsov, I., Smogór, H., Mizeracki, J.,
?ojkowski, W., 2021. Microstructure
and mechanical properties of inverse nanocomposite made from polylactide and
hydroxyapatite nanoparticles. Materials, Volume 15(1), p. 184
Rezwan, K., Chen,
Q.Z., Blaker, J.J., Boccacini, A.R., 2006. Biodegradable and bioactive porous polymer/inorganic composite
scaffolds for bone tissue engineering. Bomaterias. Volume 27(18),
pp. 3413–3431
Suchanek, W.,
Yoshimura, M., 1998. Processing and
properties of hydroxyapatite-based biomaterials for use as hard tissue
replacement implants. Journal of Materials Research, Volume
13(1), pp. 94–117
Tomozawa, M., Hiromoto, S., 2011.
Microstructure of hydroxyapatite- and
octacalcium phosphate-coatings formed on magnesium by a hydrothermal treatment
at various pH values. Acta Materialia, Volume 59(1), pp. 355–363
Tripathi, G., Basu, B., 2012. A
porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical
and biological evaluations. Ceramics
International, Volume 38(1), pp. 341-349
Xin, R., Ren,
F., Leng, Y., 2010. Synthesis and
characterization of nano-crystalline calcium phosphates with EDTA assisted
hydrothermal method. Materials & Design, Volume 31(4), pp.
1691–1694
Zhou, H., Lee, J., 2011. Nanoscale hydroxyapatite particles for bone
tissue engineering. Acta Biomaterialia, Volume 7(7), pp. 2769–2781
