Forward Osmosis to Concentrate Lithium from Brine: The Effect of Operating Conditions (pH and Temperature)
Corresponding email: bayupetrus@ugm.ac.id
Published at : 20 Jan 2022
Volume : IJtech
Vol 13, No 1 (2022)
DOI : https://doi.org/10.14716/ijtech.v13i1.4371
Sutijan, Wahyudi, S., Ismail, M.F., Mustika, P.C.B., Astuti, W., Prasetya, A., Petrus, H.T.B.M., 2022. Forward Osmosis to Concentrate Lithium from Brine: The Effect of Operating Conditions (pH and Temperature). International Journal of Technology. Volume 13(1), pp. 136-146
| Sutijan Sutijan | 1. Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No. 2 Kampus UGM Bulaksumur, D.I. Yogyakarta 55281 |
| Satrio Wahyudi | Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No. 2 Kampus UGM Bulaksumur, D.I. Yogyakarta 55281, I |
| Muhammad Fadlil Ismail | Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No. 2 Kampus UGM Bulaksumur, D.I. Yogyakarta 55281, I |
| Pra Cipta Buana mustika | Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No. 2 Kampus UGM Bulaksumur, D.I. Yogyakarta 55281, I |
| Widi Astuti | Research Unit for Mineral Technology, National Research and Innovation Agency (BRIN), Jl. Ir. Sutami Km. 15, Tanjung Bintang, Lampung Selatan, Lampung 35361, Indonesia |
| Agus Prasetya | 1. Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No. 2 Kampus UGM Bulaksumur, D.I. Yogyakarta 55281 |
| Himawan Tri Bayu Murti Petrus | 1. Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Universitas Gadjah Mada, Jalan Grafika No. 2 Kampus UGM Bulaksumur, D.I. Yogyakarta 55281 |
The development of
battery technology is the driving force behind the increasing demand for
lithium, which has resulted in a decreased supply of lithium in the market and
continues to be a challenge for the industry. In response to these conditions,
the development of lithium recovery technology continues, and there is a search
for sources of lithium that are easier to recover. One source of lithium that
has the potential to be processed is geothermal brine using forward osmosis
technology. The aim of this study was to determine the best operating
conditions for forward osmosis as a substitute for conventional evaporation
methods. The parameters to be optimized included pH and operating temperature.
The flow rate in the forward osmosis process was controlled by two litres per
hour (LPH), while the concentration of the draw solution was 6M. The operating
temperature variations used were 40oC, 35oC, and 30oC, while the pH variations used were 7, 6, and 5.
The best results were achieved at a pH of 5 with a temperature of 40oC. Apart from these operating conditions, the
activity model (the Pitzer equation) showed superior results compared to the
simple model (the Van’t Hoff equation), explaining the forward osmosis
phenomenon.
Battery; Forward osmosis; Geothermal brine; Lithium; Optimization
Lithium is one of the crucial elements because of its role in various fields, especially energy, industry, pharmaceuticals, manufacturing, and the economic sector (Hamzaoui et al., 2003). In the energy sector, increased production and electric car technology developments are predicted to be the main drivers of the increased demand for lithium. The growth in electric car production is expected to require a major market share in lithium in the 21st century (Gruber et al., 2011; Li et al., 2018). Data show that up to this point, the highest lithium consumption is still dominated by the battery production sector, and this will continue to increase over time (Naumov and Naumova, 2010). The demand for lithium in 2015 reached 173,000 tons and continues to increase (Martin et al., 2017).
Lithium on earth is found in more than 150 minerals, clay, continental brine, geothermal brine, and seawater. Hydrometallurgical lithium recovery from minerals and clays is economically less profitable than that from the brines and has the potential to cause pollution due to the use of large quantities of complex chemicals (Swain, 2017). Usually, the concentration of lithium in seawater is very low at around 0.17 ppm, while geothermal brine ranges from 0–100 ppm. Therefore, geothermal brine can be classified as a promising source of lithium availability to be commercialized economically and technically. Setiawan et al. researched lithium from geothermal brine in Indonesia. The research results show that geothermal brine from Dieng, Central Java contains lithium at an average of close to 40 ppm (Setiawan et al., 2019). Lithium recovery technology from geothermal brine is the most widely used method of evaporation (Flexer et al., 2018). However, this method tends to have many disadvantages, such as long processing times, a required area, and erratic processing energy. To deal with this problem, several methods have been developed for lithium recovery, one of which is forward osmosis.
The driving force behind forward osmosis comes from the difference in osmotic pressure between the feed and the draw solution, which results from the difference in concentration. (Cath et al., 2006). The forward osmosis process does not require high pressure like reverse osmosis (RO) (Utami et al., 2015; Desiriani et al., 2017; Suprapto et al., 2020) or a high temperature. As a result, the membrane’s fouling rate is low, and the energy value and costs required for cleaning are low (Holloway et al., 2007; McGinnis and Elimelech, 2007). The recovery of lithium from geothermal brine is carried out by drawing a quantity of water from the feed fluid body across the membrane to the draw solution fluid body (Wang et al., 2014; Awad et al., 2019). The process of displacement occurs naturally and spontaneously until it reaches equilibrium. Meanwhile, the purpose of this research is to determine the best operating conditions for the forward osmosis process to concentrate lithium from geothermal brine—a process that is feasible to continue at the commercialization stage.
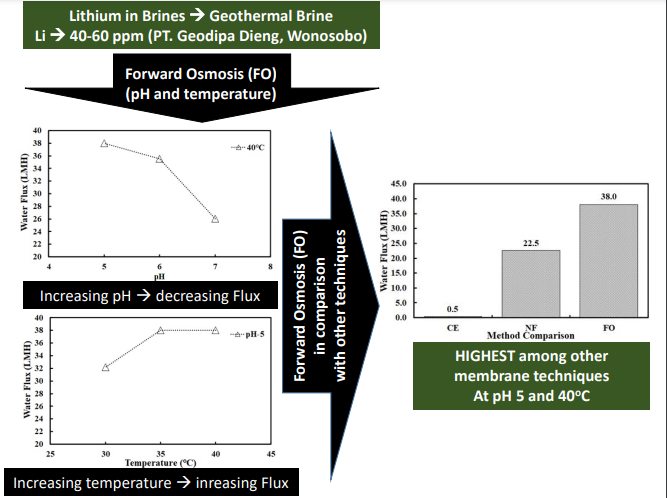
The research results generally show that a
temperature increase in the forward osmosis process results in an increase in
water flux. This condition is related to the influence of temperature on
density, viscosity, diffusivity, and others. Conversely, an increase in pH in
the forward osmosis process tends to decrease the water flux; this is related
to the charged membrane’s property, resulting in a tendency to better
separation. Meanwhile, the comparison between experiments and modeling shows
that the ICP and ECP phenomena’s occurrence plays a major role during the
forward osmosis process. The best conditions of the research were obtained at a variation of pH 5
with a process temperature of 40oC, and the ratio of normalized
concentrations reached 20 times the initial concentration. This potential is also evidenced by the high
water flux value in the lithium recovery process using an FO of around 38 LMH. Further research will include the effect of
the ratio of lithium in the feed solution, the effect of the concentration of
the draw solution, and the pilot scale model.
The authors are grateful for the support of Gadjah Mada University and the collaboration of the Research and Development Division for Mineral Technology Lampung, National Research and Innovation Agency (BRIN) for the laboratory facilities used in the research. Highly appreciation is also delivered to the Ministry of Higher Education for the financial support to conduct this research.
Awad, A.M., Jalab, R., Minier-Matar, J.,
Adham, S., Nasser, M.S., Judd, S.J., 2019. The Status of Forward Osmosis
Technology Implementation. Desalination,
Volume 461, pp. 10–21
Bhinder, A., Shabani, S., Sadrzadeh, M.,
2018. Effect of Internal and External Concentration Polarizations on the
Performance of Forward Osmosis Process. Osmotically Driven Membrane
Processes - Approach, Development and Current Status. IntechOpen
Cath, T.Y., Childress, A.E., Elimelech, M.,
2006. Forward Osmosis: Principles, Applications, and Recent Developments. Journal of Membrane Science, Volume 281(1–2),
pp. 70–87
Desiriani, R., Kresnowati, M.T.A., Wenten,
I., 2017. Membrane-Based Downstream Processing of Microbial Xylitol Production.
International Journal of Technology, Volume 8(8), pp. 1393–1401
Flexer, V., Baspineiro, C.F., Galli, C.I.,
2018. Lithium Recovery from Brines: A Vital Raw Material for Green Energies
with a Potential Environmental Impact in its Mining and Processing. Science of the Total Environment, Volume
639, pp. 1188–1204
Ghiu, S.S., Carnahan, R., Barger, M., 2002.
Permeability of Electrolytes Through a Flat RO Membrane in a Direct Osmosis
Study. Desalination, Volume 144(1–3),
pp. 387–392
Gruber, P.W., Medina, P.A., Keoleian, G.A.,
Kesler, S.E., Everson, M.P., Wallington, T. J., 2011. Global Lithium
Availability: A Constraint for Electric Vehicles? Journal of Industrial Ecology, Volume 15(5), pp. 760–775
Hamzaoui, A.H., M’nif, A., Hammi, H.,
Rokbani, R., 2003. Contribution to the Lithium Recovery from Brine. Desalination, Volume 158(1–3), pp.
221–224
Holloway, R.W., Childress, A.E., Dennett,
K.E., Cath, T.Y., 2007. Forward Osmosis for Concentration of Anaerobic Digester
Centrate. Water Research, Volume 41(17), pp. 4005–4014. https://doi.org/10.1016/j.watres.2007.05.054
Khraisheh, M., Dawas, N., Nasser, M.S.,
Al-Marri, M.J., Hussien, M.A., Adham, S., McKay, G., 2020. Osmotic Pressure
Estimation using the Pitzer Equation for Forward Osmosis Modelling. Environmental Technology, Volume 41(19),
pp. 2533–2545
Kim,
S.J., Kook, S., O’Rourke, B.E., Lee, J., Hwang, M., Kobayashi, Y., Suzuki, R.,
Kim, I. S., 2017. Characterization of Pore Size Distribution (PSD) in Cellulose
Triacetate (CTA) and Polyamide (PA) Thin Active Layers by Positron Annihilation
Lifetime Spectroscopy (PALS) and Fractional Rejection (FR) Method. Journal of Membrane Science, Volume
527(June 2016), pp. 143–151
Li, L., Deshmane, V.G., Paranthaman, M.P.,
Bhave, R., Moyer, B.A., Harrison, S., 2018. Lithium Recovery from Aqueous
Resources and Batteries: A Brief Review. Johnson
Matthey Technology Review, Volume 62(2), pp. 161–176
Martin, G., Rentsch, L., Höck, M., Bertau,
M., 2017. Lithium Market Research – Global Supply, Future Demand and Price
Development. Energy Storage Materials,
Volume 6, pp. 171–179
McCutcheon, J.R., Elimelech, M., 2006.
Influence of Concentrative and Dilutive Internal Concentration Polarization on
Flux Behavior in Forward Osmosis. Journal
of Membrane Science, Volume 284, pp. 237–247
McCutcheon, J.R., McGinnis, R.L., Elimelech,
M., 2006. Desalination by Ammonia-Carbon Dioxide Forward Osmosis: Influence of
Draw and Feed Solution Concentrations on Process Performance. Journal of Membrane Science, Volume 278(1–2),
pp. 114–123
McGinnis, R.L., Elimelech, M., 2007. Energy
Requirements of Ammonia-Carbon Dioxide Forward Osmosis Desalination. Desalination, Volume 207(1–3), pp.
370–382
Monjezi, A.A., Mahood, H.B., Campbell, A.N.,
2017. Regeneration of Dimethyl Ether as a Draw Solute in Forward Osmosis by
Utilising Thermal Energy from a Solar Pond. Desalination,
Volume 415, pp. 104–114
Naumov, A.V., Naumova, M.A., 2010. Modern
State of the World Lithium Market. Russian
Journal of Non-Ferrous Metals, Volume 51(4), pp. 324–330
Park, S.H., Kim, J.H., Moon, S.J., Jung,
J.T., Wang, H.H., Ali, A., Quist-Jensen, C.A., Macedonia, F., Drioli, E., Lee,
Y.M., 2020. Lithium Recovery from Artificial Brine using Energy-Efficient
Membrane Distillation and Nanofiltration. Journal
of Membrane Science, Volume 598, https://doi.org/10.1016/j.memsci.2019.117683
Pitzer, K.S., 1973. Thermodynamics of
Electrolytes. I. Theoretical Basis and General Equations. Journal of Physical Chemistry, Volume 77(2), pp. 268–277
Setiawan, F.A., Rahayuningsih, E., Petrus,
H.T.B.M., Nurpratama, M.I., Perdana, I., 2019. Kinetics of Silica Precipitation
in Geothermal Brine with Seeds Addition: Minimizing Silica Scaling in a Cold
Re-Injection System. Geothermal Energy,
Volume 7(1), pp. 1–16
Suprapto, S., Gotoh, T., Humaidah, N.,
Febryanita, R., Firdaus, M.S.i., Ningrum, E.O., 2020. The Effect of Synthesis
Condition of the Ability of Swelling, Adsorption, and Desorption of
Zwitterionic Sulfobetaine-based Gel. International
Journal of Technology, Volume 11(2), pp. 299–309
Swain, B., 2017. Recovery and Recycling of
Lithium: A Review. Separation and
Purification Technology, Volume 172, pp. 388–403
Utami, T.S., Arbianti, R., Manaf, B.N., 2015.
Sea Water Desalination using Debaryomyces Hansenii with Microbial Desalination
Cell Technology. International Journal of
Technology, Volume 6(7), pp. 1094–1100
Wadekar, S.S., Vadic, R.D., 2017. Influence
of Active Layer on Separation Potentials of Nanofiltration Membranes for
Inorganic Ions. Environmental Science and
Technology, Volume 51, pp. 5658–5665
Wang, W., Zhang, Y., Esparra-Alvarado, M.,
Wang, X., Yang, H., Xie, Y., 2014. Effects of pH and Temperature on Forward
Osmosis Membrane Flux using Rainwater as the Makeup for Cooling Water Dilution.
Desalination, Volume 351, pp. 70–76
Yip, N.Y., Tiraferri, A., Phillip, W.A.,
Schiffman, J.D., Elimelech, M., 2010. High Performance Thin-film Composite Forward
Osmosis Membrane. Environmental Science & Technology, Volume 44(10),
pp. 3812–3818
Zhang, X., Li, Q., Wang, J., Li, J., Zhao, C., Hou, D., 2017.
Effects of Feed Solution pH and Draw Solution Concentration on the Performance
of Phenolic Compounds Removal in Forward Osmosis Process. Journal of Environmental Chemical Engineering, Volume 5(3), pp.
2508–2514
