Preparation and Characterization of Curcumin-Based Coating Material on Co-Cr Alloy
Corresponding email: budyuls@ugm.ac.id
Published at : 25 Jan 2024
Volume : IJtech
Vol 15, No 1 (2024)
DOI : https://doi.org/10.14716/ijtech.v15i1.3588
Roza, F.N., Herliansyah, M.K., Setianto, B.Y., Githanadi, B., 2024. Preparation and Characterization of Curcumin-Based Coating Material on Co-Cr Alloy. International Journal of Technology. Volume 15(1), pp. 18-27
| Faizatin Nadya Roza | Biomedical Engineering Study Program, The Graduate School of Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia |
| Muhammad Kusumawan Herliansyah | Department of Mechanical and Industrial Engineering, Faculty of Engineering, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia |
| Budi Yuli Setianto | Department of Cardiology and Vascular Medicine, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia |
| Brillyana Githanadi | Biomedical Engineering Study Program, The Graduate School of Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia |
Co-Cr; Coating; Curcumin; PLLA
Coronary heart disease (CHD) is one of the most
considerable health problems which can be the leading cause of death worldwide (Khan et al., 2020). It is
caused by the narrowing of blood vessels or other
abnormalities, primarily due to plaque formation known as atherosclerosis,
which can lead to a heart attack. Atherosclerosis can be overcome by installing
a stent. Stent aims to support the coronary vessel wall so it does not easily
recoil after being dilated with a balloon (Grabow et al., 2010). It
is mostly made of metal such as stainless steel, platinum alloys-chromium (Jorge and Dubois, 2015), or
cobalt-chromium (Co-Cr), then formed into a small pipe, originally known as
bare metal stent (BMS). Co-Cr alloys have high density, which is
advantageous to radio-opacity, and have high elastic modulus, which limits
recoil, and tensile strength properties that allow stent designs with thinner
struts (Poncin et al., 2005).
Besides
the promising role of Co-Cr stents for minimization of coronary remodeling,
restenosis risk remains a critical concern. Restenosis could occur due to blood
clotting, called thrombosis, which can lead to the proliferation of vascular
smooth muscle cells (VSMC) (Foerst et
al., 2013). Besides the promising role of Co-Cr stents for the
minimization of coronary remodeling, restenosis risk remains a critical
concern. Restenosis could occur due to blood clotting called thrombosis,
leading to the proliferation of vascular smooth muscle cells (VSMC) (Foerst et al., 2013).
The
VSMC proliferation and intracellular matrix synthesis in response to
stent-implanted inflammatory reaction are broadly believed as the major
mechanisms of restenosis, and it needs a drug to prevent it (Bennet and
Michael, 2001). However, orally administered drugs may have inadequate
local drug concentration and can cause toxic reactions from excessive drug
doses. Therefore, a drug delivery system is needed to control drug release (Imani et al.,
2022). The drug delivery system works by preserving the drug
and restraining the drug release; therefore, the drug can reach its action site
appropriately by enhancing and/or reducing the drug circulation (Barleany et al.,
2020). To deal with this issue, drug coating stents which are
globally known as drug-eluting stents (DESs), become the answer by releasing
pharmacological agents to inhibit the response of restenosis (Bennet and Michael,
2001).
Previously,
DES, which releases anti-proliferative drugs such as sirolimus (rapamycin) and
paclitaxel with synthetic polymer
coatings, has opened up a new paradigm for the treatment of in-stent restenosis
(ISR), known as first-generation DES. Advantageously, these drug-coating stents
can provide luminal scaffolding that eventually eliminates the recoil and
remodeling of vascular coronary. However, the released drug from the coating
can achieve high local drug concentration, cellular proliferation prevention,
or thrombus formation (Waksman et al., 2006).
Furthermore, the residual synthetic polymer coating remains in the body, which
may lead any complications such as an over-inflammatory response and neointimal hyperplasia at the implant
site (Ranade
et al., 2004). To avoid these unavailing effects, it is
urgent to build a drug-coating stent with a biodegradable and biocompatible
coating. One kind of biodegradable polymer that has good mechanical properties
is Poly-(L lactic acid) (PLLA). This polymer is known to be the most desirable
biocompatible and biodegradable polymer obtainable from starch in a high yield (Ni’mah et al., 2019). PLLA is
widely used in biomedical devices such as orthopedic surgery and other surgery
fields where bioresorbable sutures are needed, as well as used for
drug-delivering implants (Zilberman
and Eberhart, 2006). Because of its superior mechanical strength,
PLLA can be a good candidate as a drug carrier for stent coatings.
Curcumin
is a polyphenolic compound commonly
derived from the dried rhizomes of Curcuma
longa L. (Basile
et al., 2009), presents low intrinsic toxicity and shows a
wide spectrum of pharmacological properties, including anti-oxidation,
anti-inflammation, anti-thrombus and anti-proliferation activities (Chen et al.,
2015). These promising characteristics suggest that curcumin could be applicable as a
therapeutic agent for DES.
By considering these biomaterials, the
preparation of the curcumin-based coating using Poly-(L lactic acid) (PLLA) as
the drug material, along with the in vitro characteristics in this study, were
reported. To our knowledge, the preparation of curcumin coating by using PLLA to be coated on Co-Cr alloy has not
been investigated.
2.1. Materials
The material used in this study was Co-Cr alloy
(Dentaurum GmbH & Co. KG, Ispringen, Germany). The composition (wt%), as
provided by the manufacturer, was 60.5% cobalt
(Co), 28% chromium (Cr), 1.5% silicon (Si), 9% tungsten (W), other elements were manganese (Mn), nitrogen
(N), niobium (Nb), and iron (Fe) were less than 1%, and free
from nickel (Ni), beryllium (Be), and gallium (Ga). The coating material was curcumin (Merck KGaA, Darmstadt,
Germany) that had a purity above 96% and PLLA (30% wt. in H2O)
(L1875–Sigma Aldrich, Massachusetts, US). All other reagents used in this
research were analytical grade.
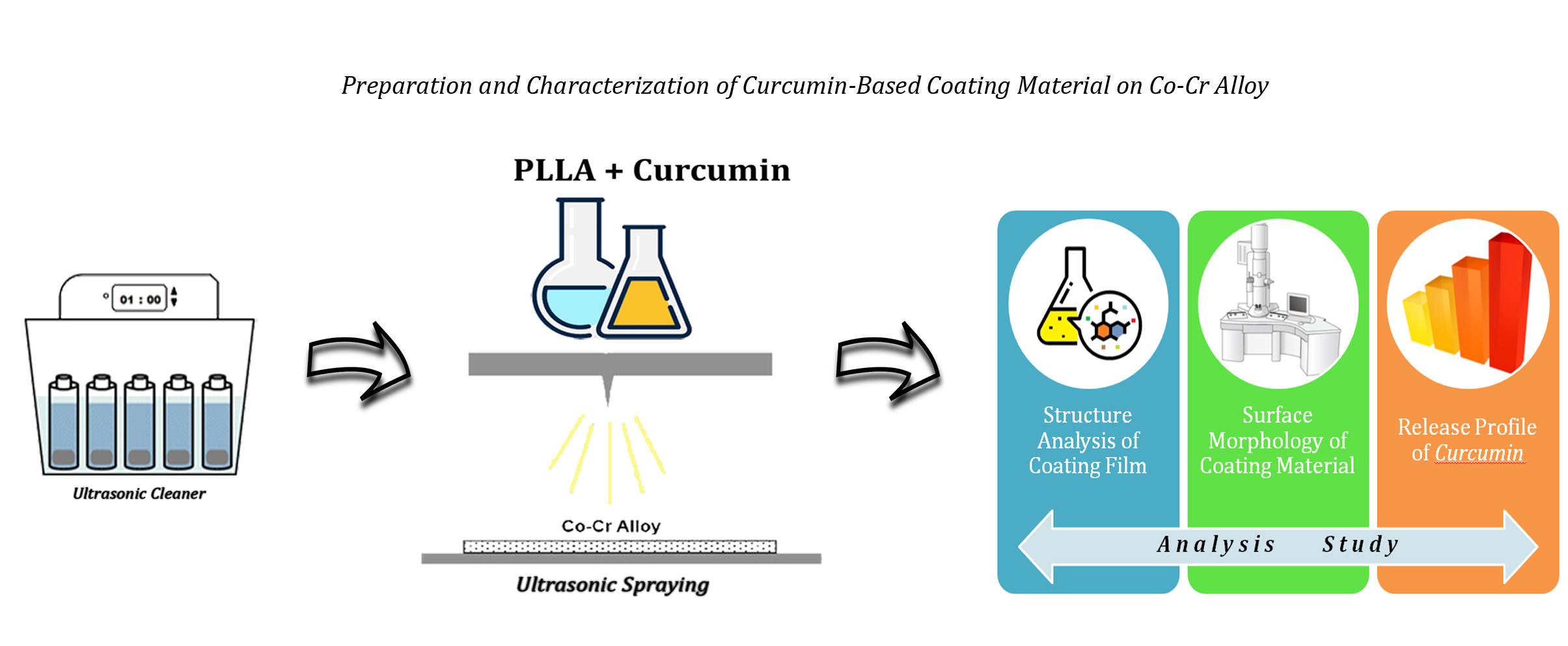
2.2. The Curcumin-based Coating Preparation
Firstly, the Co-Cr tube was shaped into a platted disk by a mold casting
machine. The materials were polished and then were cleaned ultrasonically with
acetone (Merck KGaA, Darmstadt, Germany), ethyl alcohol (Merck KGaA, Darmstadt,
Germany), and distilled water sequentially. The cleaned materials were
preserved under a vacuum to evaporate the residual water (Pan et al., 2006). Next,
curcumin, which was previously dissolved in absolute ethanol at different
concentrations, was mixed with 1% wt. of PLLA using a homogenizer for at least
3 minutes. The solutions were then sprayed onto cleaned materials using the
ultrasonic spraying method, with each sample sprayed for at least 1 minute. It
was remarked parenthetically that the solutions sprayed to all samples were
about 0.5 ml with about 3µm thickness of the coating severally. Those sprayed
mass of coating on the sample surface could be obtained by calculating the
solution concentration. Three coating concentrations of curcumin were prepared at low concentration (~62.5 µg), moderate
concentration (~125 µg), and high concentration (~250 µg). There were two kinds
of control samples prepared. The first one was with only polymer, and the
second one was with curcumin with the
same concentration and the same method of coating.
Figure 1 Schematic of Material Preparation using
Ultrasonic Cleaner and Ultrasonic Spraying
2.3. Fabrication of the coating films
To cast a thin polymer film, a one wt.% solution PLLA
was prepared by dissolving the solution in ethyl acetate (Merck KGaA,
Darmstadt, Germany), then placed on a cleaned watch glass dish 10 cm. The films
were slowly dried in hot air until the solvent evaporated to obtain the films.
The films were then preserved to evaporate the residual solvent. The curcumin
coating films were prepared in two different ways. First, curcumin with high
concentration dissolved in ethanol was mixed with dissolved PLLA in ethyl acetate,
and the second was dissolved curcumin in ethanol only. Both of them were
evaporated and were treated in the same manner as such polymer films.
2.4.
Structure analysis of the coating films
The structure of PLLA, curcumin, and curcumin/PLLA
films was analyzed by the FTIR system (FTIR type SHIMADZU IR-Prestige 21,
Japan) to determine the chemical compounds. Further, curcumin powder was also
examined for additional control data. The scanning of the FT-IR range equipped
with an attenuated total reflectance accessory for wavenumbers from 4,000 cm-1
to 300 cm-1, approximately 20 scans were performed for each film
with 1 cm resolution.
2.5.
Morphology of curcumin-based coating
The surface morphology of curcumin-based and PLLA
coatings was investigated using analytical scanning electron microscopy
(SEM-EDX JEOL JSM-6510LA, JEOL Ltd., Japan), operated at SEI with 20 kV
accelerating voltage. In addition, energy-dispersive X-ray spectroscopy (EDS)
analysis was also performed to determine the abundance of the specific chemical
elements of each sample.
2.6.
Curcumin
Release Profile
The release profile of the curcumin from the samples
was examined in vitro by immersing each sample in a medium consisting of
phosphate-buffered saline (PBS, pH=7.4) (Invitrogen by Thermo Fisher
Scientific, Massachusetts, US) with 10% ethanol at 37OC because the
solubility limit of the curcumin in water that makes it difficult to be
investigated in buffer (Alexis et al., 2004). At specific intervals, the medium of the PBS solution
was removed completely and replaced with the fresh medium. The removed medium
was then measured using a UV-Vis spectrophotometer (VWR®V V-1200,
UV–1600PC Spectrophotometer UV-Vis, England) at wavelength 425 nm to determine
the amount of released curcumin. The results were obtained accumulatively in
micrograms (µg) and percentages (%) of released curcumin. Moreover, in order to
investigate the drug release mechanism, it was applied by using the equation of
Ritger & Pappas (see Equation 1) as below:
where Mt was the amount of curcumin released at the time (t), was the total amount of curcumin, and k was a constant parameter of the
release exponent (Kharaziha et al., 2015).
3.1. FTIR Analysis
FTIR spectra of curcumin, PLLA, curcumin coating film, and curcumin/PLLA coating film are shown in Figure2. A transmission band related to curcumin was observed at 3448 cm-1. This was attributable to the stretching vibrations of the phenolic O-H group. However, this band could not be distinguished from other peaks in curcumin coating film and so curcumin/PLLA coating film. A sharp peak was seen at 1597 cm-1, corresponding to the stretching of the C = C bond of the benzene ring. Another sharp peak was seen at 1512 cm-1. It was related to the olefin bending vibrations of the C-H bond to the benzene ring of curcumin. Those peaks were shifted to 1620 cm-1 and 1520 ccm-1, respectively, in curcumin coating film, as well in curcumin/PLLA coating film to 1589 cm-1 and 1517 cm-1, respectively. Further, the peak from pure curcumin at 817 cm-1 and 1280 cm-1, assigned for vibration of C–O in –C–OCH3 of the phenyl ring, were shifted and only detected at 1288 cm-1 in curcumin coating film and at 1285 cm-1 in curcumin/PLLA coating film.
Figure 2 Result of
the FTIR Analysis of Pure Curcumin and PLLA, Curcumin Film, and Curcumin/PLLA
film
Similarly, with the curcumin-related band, a
valley-like peak at 3425 cm-1 was seen in PLLA film. This was
probably ascribable to hydroxyl stretching OH bending. Other characteristic peaks of PLLA and their shift in
curcumin/PLLA were also identified. A blunt peak at 1628 cm-1 is
assigned as carbonyl stretching C=O in the –CO–O– group of PLLA and was
observed vaguely at 1728 cm-1 at curcumin/PLLA. Another hollow-like
peak was seen at 1180 cm-1. This corresponds to the stretching
vibrations of the symmetric CH bending in the –CH–O– chains of PLLA, and it
shifted to 1141 cm-1 in the case of curcumin/PLLA. Additionally, a
mountainous triplet peak at 1033; 956; and 879 cm-1 that were
ascribable to the C–O bond vibration in –CO–O– group in PLLA chains shifted
accordingly to 1026; 934; and 848 cm-1 respectively in
curcumin/PLLA.
Based on
the peak shifts shown in the FTIR data (Figure 2), it can be confirmed that
curcumin and PLLA were adequately bound together. Meanwhile, the peak at 879 cm-1 in PLLA, which was ascribable to the C–O bond vibration in the –CO–O– group in
PLLA chains, shifted to 848 cm-1 in curcumin/PLLA. That shifting could be confusing with the vibrations
of the phenyl ring C–O in –C–OCH3 from curcumin. Nevertheless, this confusion can lead to the certainty
that curcumin and PLLA were perfectly
blended. Furthermore, a peak at 1628 cm-1 that was identified as
carbonyl stretching C=O in the –CO–O– a group of PLLA then shifted to 1728 cm-1 at curcumin/PLLA, indicating weak
hydrogen bond formation between the carbonyl group of PLLA and the hydroxyl
group of curcumin. This finding was
reinforced by the peak shift of the symmetric CH bending of PLLA in curcumin/PLLA, from 1180 cm-1 to 1141 cm-1. C–H groups that showed symmetric CH bending were the
neighboring groups to C = O in PLLA. The changes in vibrational frequency indicated
by the peak shift were probably the consequence of interaction between the C = O
group of PLLA with the O–H group of curcumin.
3.2. SEM-EDS Analysis
SEM images were taken
on the top surface where curcumin, curcumin/PLLA, and PLLA were coated on
Co-Cr Alloy. Those images are shown in Figure 3, Figure 4, and Figure 5 to
evaluate the morphology of the coating. There are two types of analyzed samples
for SEM, first, the samples that were only coated with curcumin, curcumin/PLLA,
and PLLA on Co-Cr Alloy, and the second were the coated samples with release
treatment with PBS (phosphate buffer saline) for about 20 hours.
Figure 3 SEM Images of curcumin coated before (A) and after (B)
PBS treatment
Figure 4 SEM Images of PLLA
coated before (C) and after (D) PBS treatment
Figure 5 SEM Images of curcumin/PLLA coated before (1, 3)
and after (2, 4) PBS treatment
A
considerably smooth surface was observed on the surface where the sample was
coated with PLLA only. It could be indicated that the PLLA was coated perfectly
with Co-Cr. However, after giving the release treatment with PBS, it was shown
that the PLLA surface formed some cuboid shape particles that claim the PLLA
surface has a deformation on its particles. On the other hand, A rough surface
with circular shaped particles was seen on a sample that was coated with curcumin only. It is indicated the distribution
of the curcumin particle on the
material surface. Further, a fractured surface was then observed after the
coating was treated with PBS. At fractured surface condition shows that the curcumin was released and formed a
deformation on the surface. Surprisingly, on the SEM images of the
curcumin/PLLA coated surface revealed the presence of numerous tubular-shaped
particles on the surfaces. Those deformations on curcumin/PLLA particles could be caused by the adsorption of the curcumin onto PLLA. curcumin/PLLA coating surface after treatment with PBS.
In the
other images of the curcumin/PLLA
coatings, there was a sloughed area on the coated sample surface which was
treated by PBS. Those indicated that the curcumin/PLLA
coating which was treated by PBS, was released well at almost one day of
releasing time. In addition, it can be vaguely seen that there were some
shrinking particles on the Table 1.
Table 1 Element composition (wt%), measured using
energy-dispersive spectroscopy (EDS), of the surface of the cobalt-chromium
(CoCr) alloy coated with PLLA, Curcumin,
and Curcumin/PLLA
|
Sample Treatment |
Element Composition Mass (wt.%) |
| ||||||
|
Co |
Cr |
W |
Si |
C |
H |
O | ||
|
PLLA Coating |
53 |
26 |
8 |
1 |
9 |
0 |
2 | |
|
Curcumin Coating |
40 |
19 |
6 |
1 |
28 |
0 |
6 | |
|
Curcumin/PLLA Coating |
39 |
20 |
7 |
1 |
27 |
0 |
5 | |
The result of the surface topography of the coated
specimens analyzed using energy-dispersive spectroscopy (EDS) was barely
perceptible in the spectrum images of EDS analysis (data was not shown). In
spite of the spectra analysis of the EDS, the result of the element composition
mass of the specimens had some differences discernibly. On the top surface of
the PLLA coated specimen, the composition mass of the Co-Cr alloy such as Co,
Cr, W, and Si was the highest among other coating treatment samples. It could
be argued that the PLLA coating film on Co-Cr alloy only slightly affect the
element composition of the material. However, on the curcumin-only coating and curcumin/PLLA
coating, there were obviously increments in the composition of the coating
elements. Among the three specimens, the curcumin/PLLA
coating sample had the lowest amount of mass of the Co-Cr element composition,
whereas the curcumin coating sample
had the most sizeable amount of coating composition of elements. The coating
elements, which were C, H, and O, were adapted from curcumin (C21H20O6) and PLLA (C3H6O3)
3.3. The Release Profile of Curcumin
Figure 6 In vitro release profile of curcumin from three concentration types
of coated materials; the cumulative amount of curcumin
Figure 7 In vitro release
profile of curcumin from three
concentration types of coated materials; the percentage weight of released curcumin
The release of the
drug from the matrix paired with the polymer begins with the presence of an
initial burst release (Dinarvand et al., 2005). Therefore, tests were carried out in two different
types of time intervals, the fast release period in the first hour for 8 hours
after treatment with PBS, then followed by a moderate-slow release period of
observation for 14 days. Based on the results in Figure 6, all specimens coated
with curcumin/PLLA in each treatment
showed a nearly linear controlled-release profile with no significant burst
release observed during the first hour period up to 14 days of the test.
Theoretically, the controlled release of anti-restenosis drugs is required to
prevent the migration and proliferation of vascular smooth muscle cells (VSMC)
for at least one month after stenting implantation (Puranik, Dawson, and Peppas,
2013). From the curcumin release profile graph in Figure 6, the average release of
the controlled rate at low concentrations is ±1.42 µg per day (2.27% wt.), at
moderate concentrations ±2.21 µg per day (1.76% wt.), and ±3.03 µg per day
(1.21%) at high concentrations. Furthermore, based on the average rate of curcumin release, it could be decided
that the duration of curcumin-controlled
release is ±44 days for curcumin/PLLA
low concentration, ±57 days at moderate concentration, and for ± 82 days at curcumin/PLLA high concentration. All of
the concentrations overall met the criteria for drug release for at least 30
days (one month). Additionally, the Ritger and Peppas equation (Equation 1) was
also applied to evaluate the mechanism of curcumin
release (Kharaziha et al., 2015). The results showed that the release of curcumin in the curcumin/PLLA
coating in this study occurred through a diffusion mechanism. The treatment of
replacing the release medium at successive day intervals resulted in a
significant gradient of curcumin
concentration between curcumin/PLLA
with the release medium, causing the formation of barely constant linear lines
from the diffusion of the drug, as shown in Figure 6. Diffusion-sustained drug
delivery systems can arrange the delivery of drugs for several weeks and give a
special capability to control the kinetics of drug release (Acharya and
Park, 2006). In addition, the diffusion of
drug molecules through the polymer matrix depends on the solubility of the drug
in the polymer, as well as the medium surrounding the drug as the diffusion
coefficient and concentration gradient of the drug to the polymer. Therefore,
it can provide an indication that if the diffusion of curcumin is faster than the degradation of the polymer matrix, it
means that the mechanism of releasing curcumin
occurs through the diffusion mechanism (Mohammadi et al.,
2019).
In conclusion, based on the
results obtained in this research suggested that curcumin-based coating had the potential to become a coating
material for improving drug-eluting stents. Curcumin
could be blended with PLLA at the molecular level as coating material using
ultrasonic spraying as shown by SEM images. In addition, curcumin could be indicated to disperse in the PLLA matrix
according to FTIR results. Curcumin also can be potential material for
drug eluting stents as it showed the mildly sustained release profile without
any apparent burst release. The slightly drawback of curcumin release
profile which is needed to be reviewed is to find the moderate amount of
curcumin concentration that can be released no more than 30 days in order to
release at the appropriate duration.
This research could be finished and succeeded with
full support from Universitas Gadjah Mada Grants that are Final Assignment
Recognition Grant (RTA 2019) [3142/UN1/DITLIT/DIT-LIT/LT/2019] and University
Excellence Development Research Grant (PPUPT 2018) [1993/UN1/DITLIT/DIT-LIT/LT/2018].
Additionally, a grateful pleasure for Professor Widowati Siswomihardjo and Mr.
Ir. Alva Edy Tontowi, M.Sc., Ph.D. as the Head and Vice Head of Biomedical
Engineering Universitas Gadjah Mada.
| Filename | Description |
|---|---|
| R2-MME-3588-20230614113844.jpg | Result of the FTIR Analysis of Pure Curcumin and PLLA, Curcumin Film and Curcumin/PLLA film. (figure 2 (2) - right) |
Alexis, F., Venkatraman, S.S., Rath, S.K.,
Boey, F., 2004. In Vitro Study of Release Mechanisms of Paclitaxel and
Rapamycin from Drug-Incorporated Biodegradable Stent Matrices. Journal of Controlled Release, Volume 98(1), pp. 67–74
Barleany, D.R., Ananta,
C.V., Maulina, F., Rochmat, A., Alwan, H., Erizal, 2020. Controlled Release of
Metformin Hydrogen Chloride from Stimuli-responsive Hydrogel based on Poly(N-
Isopropylacrylamide)/Chitosan/Polyvinyl Alcohol Composite. International
Journal of Technology, Volume 11(3), pp. 511–521
Basile, V., Ferrari, E., Lazzari,
S., Belluti, S., Pignedoli, F., Imbriano, C., 2009. Curcumin Derivatives:
Molecular Basis of Their Anti-Cancer Activity. Biochem Pharmacol, Volume 78(10), pp. 1305–1315
Bennet, M.R., O'Sullivan, M.,
2001. Mechanisms of Angioplasty and Stent
Restenosis: Implications for Design of Rational Therapy. Pharmacology and Therapeutics, Volume 91(2), pp. 149–166
Chen, W., Habraken, T.C.J.,
Hennink, W.E., Kok, R.J., 2015. Polymer-Free Drug- Eluting Stents: An Overview of Coating Strategies
and Comparison with Polymer-Coated Drug-Coating Stents. Bioconjugate Chemistry, Volume 26 (7), pp. 1277–1288
Dinarvand, R., Mahmoodi,
S., Farboud, E., Salehi, M., Atyabi, F., 2005. Preparation of Gelatin
Microspheres Containing Lactic Acid—Effect of Crosslinking on Drug Release. Acta Pharmaceutica, Volume 55(1), pp. 57–67
Foerst, J., Vorpahl, M.,
Engelhardt, M., Koehler, T., Tiroch, K., Wessely, R., 2013. Evolution of
coronary stents: From bare-metal stents to fully biodegradable, drug eluting stents. Combination Products in Therapy, Volume 3(1-2), pp. 9–24
Grabow, N., Martin, D.P.,
Schmitz, K.P., Stenberg, K., 2010. Absorbable Polymer Stent Technologies for
Vascular Regeneration. Journal of
Chemical Technology & Biotechnology, Volume 85(6), pp. 744–751
Imani, N.A.C., Kusumastuti,
Y., Petrus, H.T.B.M., Timotius, D., Putri, N.R.E., Kobayashi, M., 2022.
Preparation, Characterization, and Release Study of Nanosilica/Chitosan
Composite Films. International Journal of Technology. Volume 13(2),
pp. 444–453
Jorge, C., Dubois, C.,
2015. Clinical Utility of Platinum Chromium Bare Metal Stents in Coronary Heart
Disease. Medical Devices: Evidence
and Research, Volume 8, pp. 359–367
Khan, M.A., Hashim M.J.,
Mustafa, H., Baniyas, M.Y., Al-Suwaidi, S.K.B.M., Alkatheeri, R., Alblooshi,
F.M.K., Almatrooshi, M.E.A.H., Alzaabi, M.E.H., Al-Darmaki, R.S., Lootah,
S.N.A.H., 2020. Global Epidemiology of Ischemic Heart Disease: Results from The
Global Burden of Disease Study. Cureus, Volume 12(7), p. 9349
Kharaziha, M., Fathi, M.H.,
Edris, H., Nourbakhsh, N., Talebi, A., Salmanizadeh, S., 2015. PCL-Forsterite
Nanocomposite Fibrous Membranes for Controlled Release of Dexamethasone. Journal of Materials
Science: Materials in Medicine, Volume 26(1), p. 36
Mohammadi, F., Golafshan,
N., Kharaziha, M., Ashrafi, A., 2019. Chitosan-Heparin Nanoparticle Coating on
Anodized NiTi for Improvement of Blood Compatibility and
Biocompatibility. International
Journal of Biological Macromolecules,
Volume 127, pp. 159–168
Ni'mah, H., Rochmadi, R.,
Woo, E.M., Widiasih, D.A., Mayangsari, S., 2019. Preparation and
Characterization of Poly(L-lactic acid) Films Plasticized with Glycerol and
Maleic Anhydride. International Journal of Technology, Volume
10(3), pp. 531–540
Pan, Ch.J., Tang, J.J.,
Weng, Y.J., Wang, J., Huang, N., 2006, Preparation, Characterization And
Anticoagulation of Curcumin-Eluting Controlled
Biodegradable Coating Stents, Journal
of Controlled Release, Volume
116(1), pp. 42–49
Poncin, P., Millet, C., Chevy, J., Proft, J.L.,
2004. Comparing and Optimizing Co-Cr Tubing for Stent Applications. In:
Proceedings of Materials and Processes for Medical Devices Conference, pp. 279–283
Puranik, A.S., Dawson,
E.R., Peppas, N.A., 2013. Recent Advances in Drug Eluting Stents. International
Journal of Pharmaceutics. Volume 441(1-2), pp. 665– 679
Ranade, S.V.,
Miller K.M., Richard R.E., Chan, A.K., Allen, M.J., Helmus, M.N.,
2004. Physical Characterization of Controlled Release of Paclitaxel from
the TAXUS™ Express 2™ Drug-Eluting Stent. Journal of Biomedical Materials Research. Volume 71(4), pp. 625–634
Sternberg, K., Kramer, S.,
Nischan, C., Grabow, N., Langer, T., Hennighausen, G., Schmitz, K.-P., 2007. In
Vitro Study of Drug- Eluting Stent
Coatings Based on Poly(L-lactide) Incorporating Cyclosporine A–Drug Release,
Polymer Degradation and Mechanical Integrity. Journal of Materials Science: Materials in Medicine, Volume 18(7), pp. 1423–143
Waksman, R., Pakala, R.,
Baffour, R., Hellinga, D., Seabron, R., Kolodgie F., Virmani R., 2006. Optimal
Dosing and Duration of Oral Everolimus to Inhibit In-Stent Neointimal Growth in
Rabbit Iliac Arteries. Cardiovascular
Revascularization Medicine, Volume 7(3),
pp. 179–184
Zilberman, M., Eberhart,
R.C., 2006. Drug-Eluting Bioresorbable
Stents for Various Applications.
Annual Review of Biomedical
Engineering. Volume 8, pp. 153–180
